Please refer to Surface Chemistry HOTs Class 12 Chemistry provided below with Surface Chemistry. All HOTs for Class 12 Chemistry with answers provided below have been designed as per the latest syllabus and examination petter issued by CBSE, NCERT, KVS. Students of Standard 12 Chemistry should learn the solved HOTS for Class 12 Chemistry provided below to gain better marks in examinations.
Surface Chemistry Class 12 Chemistry HOTs
MULTIPLE CHOICE QUESTIONS
Question. What is the emulsifer in milk?
(a) albumin
(b) soap
(c) gelatin
(d) caesin
Answer
B
Question. Bredig’s are method can not be used for the preparation of colloidal sol of
(a) Cu
(b) Mg
(c) Ag
(d) Na
Answer
B,D
Question. Which of the following has least coagluating value for positive sol?
(a) Cl–
(b) SO42–
(c) PO4–3
(d) [Fe(CN)6]–4
Answer
D
Question. Which can adsorb larger volume of hydrogen gas?
(a) Colloidal solution of platinum
(b) finely divided nickel
(c) finely divided platinum
(d) colloidal Fe(OH)3
Answer
A
Question. Which one of the following gases will be adsorbed most easily?
(a) N2
(b) H2
(c) O2
(d) CO2
Answer
D
Question.A catalyst do not change:
(a) gibbs energy of reaction
(b) enthalpy of reaction
(c) equilibrium constant
(d) Activation energy of reaction
Answer
A,B,C
Question. The formation of micelles takes place only above:
(a) inversion temperature
(b) Boyle temperature
(c) critical temperature
(d) Kraft temperature
Answer
D
Question. Which is method of purification of colloidal solution?
(a) ultrafiltration
(b) electrodialysis
(c) bredig’s arc method
(d) dialysis
Answer
A,B,D
Question. Alums purify muddy water by:
(a) dialysis
(b) adsorption
(c) absorption
(d) coagulation
Answer
D
Question. Rate of physisorption increases with:
(a) decrease in temperature
(b) increase in temperature
(c) decrease in pressure
(d) decrease in surface area
Answer
A
Question. Which of the following is an example of associated colloid?
(a) soap in water
(b) protein in water
(c) rubber in benzene
(d) AgNO3 in water
Answer
A
Question. The colloidal system consisting of a liquid adsorbate in a solid adsorbent is termed as:
(a) aerosol
(b) foam
(c) emulsion
(d) Gel
Answer
D
Question. The coagulating power of an electrolyte for blood decrease in the order.
(a) Na+, Al+3, Ba+2
(b) PO4–3, SO4–2, Cl–
(c) Al+3, Ba+2, Na+
(d) Cl–, SO42–, PO4–3
Answer
C
Question.A colloidal solutions show:
(a) very high osmotic pressure
(b) high osmotic pressure
(c) low asmotic pressure
(d) no osmotic pressure
Answer
C
Question.Cottrell precipitator works on the principle of:
(a) distribution law
(b) addition of electrolate
(c) Le-chattelier principle
(d) Neutralisation of charge on collids
Answer
D
Question. Which of the following statements is not correct for chemisorption and physisorption?
(a) Physisorption and chemisorption are both exothermic processes.
(b) Magnitude of chemisorption is favourable at low temperature while physisorption is favourable at high temperature.
(c) Chemisorption is irreversible and physisorption is reversible.
(d) In physisorption activation energy is low while in chemisorption it is high.
Answer
B
Question. When a colloidal solution is viewed from the direction at right angles of light beam, the path of the beam is illuminated due to scattering of light. In the figure (A) and (B) are
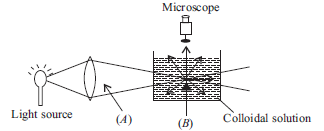
(a) A – Tyndall cone, B – Scattered light
(b) A – Scattered light, B – Tyndall cone
(c) A – Tyndall cone, B – Blind spot
(d) A – Tyndall effect, B – Tyndall cone
Answer
A
Question. Fe(OH)3 sol can be more easily coagulated by Na3PO4 in comparison to KCl because
(a) mass of Na3PO4 is more than KCl hence it is more effective than KCl
(b) phosphate ion (PO43–) has higher negative charge than Cl– ion hence are more effective for coagulation
(c) KCl is more soluble than Na3PO4 hence less effective for coagulation
(d) Na+ ions are more effective than K+ ions for coagulation.
Answer
B
Question. Which of the following curves is in accordance with Freundlich adsorption isotherm?
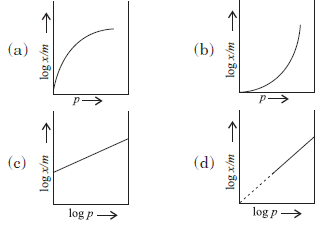
Answer
C
Question. A colloidal system in which liquid is dispersed phase and solid is dispersion medium is classified as
(a) gel
(b) sol
(c) emulsion
(d) aerosol.
Answer
A
Question. Mixing of positively charged colloidal solution with negatively charged colloidal solution brings . The decreasing order of coagulating power of Na+, Ba2+ and Al3+ for negatively charged colloidal solution is .
(a) mutual coagulation, Na+ > Ba2+ > Al3+
(b) mutual coagulation, Al3+ > Ba2+ > Na+
(c) coagulation, Na+ > Ba2+ > Al3+
(d) peptization, Al3+ > Ba2+ > Na+
Answer
B
Question. The cause of Brownian movement which is not shown by true solutions or suspensions is due to
(a) unbalanced bombardment of particles by molecules of the dispersion medium
(b) attractive forces between dispersed phase and dispersion medium
(c) larger size of the particles due to which they keep colliding and settling down
(d) conversion currents formed in the sol.
Answer
A
Question. The substances which behave as normal electrolytes at low concentration but undergo association at higher concentration and behave as colloidal solutions are called
(a) associated colloids
(b) multimolecular colloids
(c) macromolecular colloids
(d) protective colloids.
Answer
A
Question. Which of the following examples is correctly matched?
(a) Butter – gel
(b) Smoke – emulsion
(c) Paint – foam
(d) Milk – aerosol
Answer
A
Question. Which of the plots is adsorption isobar for chemisorption?
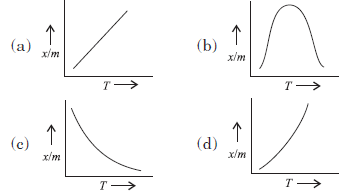
Answer
B
Question. Which of the following is not a method of removing impurities from a colloidal sol?
(a) Electrodialysis
(b) Ultrafiltration
(c) Ultra centrifugation
(d) Distillation
Answer
D
Question. Which of the following gases is least adsorbed on charcoal?
(a) HCl
(b) NH3
(c) O2
(d) CO2
Answer
C
Question. Colloidal solutions of metals like gold can be prepared when their salt solutions react with certain substances like SnCl2, formaldehyde, phenyl hydrazine, etc.
2AuCl3 + 3SnCl2 → 3SnCl4 + 2Au
sol
The above method is an example of
(a) reduction method
(b) oxidation method
(c) hydrolysis method
(d) double decomposition method.
Answer
A
Question. Which of the following statements is not correct about physiosorption?
(a) It is a reversible process.
(b) It requires less heat of adsorption.
(c) It requires activation energy.
(d) It takes place at low temperature.
Answer
C
Question. What is the role of activated charcoal in gas masks used in mines?
(a) It acts as an adsorbent for poisonous gases present in coal mines.
(b) It acts as an adsorbent for coal particles present in coal mines.
(c) It acts as a mask through which exhaled gases are diffused out.
(d) It acts as a base for scattering the light.
Answer
A
Question. A lyophobic colloid cannot be formed by
(a) mixing dispersed phase and dispersion medium
(b) chemical reactions like hydrolysis
(c) exchange of solvent
(d) peptisation.
Answer
A
Question. The term activation of adsorbent is used when
(a) adsorbing power is increased by increasing surface area by making the surface rough
(b) adsorbing power is increased by dipping the surface in acid to make it smooth
(c) adsorbing power is increased by dissolving it in water
(d) adsorbing power is decreased to reduce the extent of adsorption.
Answer
A
Question. Movement of dispersion medium under the influence of electric field is known as
(a) electrodialysis
(b) electrophoresis
(c) electroosmosis
(d) cataphoresis.
Answer
C
Question. Which of the processes is being shown in the figure?
(a) Electrodialysis
(b) Dialysis
(c) Electroosmosis
(d) Electrophoresis
Answer
A
Question. Which of the following statements does not show correct difference between adsorption and absorption?
(a) In adsorption the substance is concentrated only at the surface while in absorption it is uniformly distributed in the bulk.
(b) Adsorption is instantaneous while absorption is a slow process.
(c) A substance can be adsorbed as well as absorbed simultaneously and the process is called sorption.
(d) Only gases are adsorbed while solids and liquids are absorbed.
Answer
D
Question. Fog is an example of colloidal system of
(a) liquid in gas
(b) gas in liquid
(c) solid in gas
(d) gas in solid.
Answer
A
Question. Why is alum added to water containing suspended impurities?
(a) To make a colloidal solution.
(b) To coagulate the suspended impurities.
(c) To remove impurities of calcium and magnesium.
(d) To protect the colloidal solution from getting precipitated.
Answer
B
Question. Which of the following gases present in a polluted area will be adsorbed most easily on the charcoal gas mask?
(a) H2
(b) O3
(c) N2
(d) SO2
Answer
D
Match the column
Question. Match the column and choose correct option:
(A) Smoke P. foam
(B) Butter Q. emulsion
(C) Hair cream R. aerosol
(D) Whipped cream S. gel
(a) A–P, B–S, C–Q, D–R
(b) A–R, B–Q, C–S, D–P
(c) A–R, B–S, C–Q, D–P
(d) A–S, B–P, C–R, D–Q
Answer
C
Question.Matching Column Type
Column 1 Column 2
(A) Soap in water P. Associated colloid
(B) Starch gelatin Q. Lyoptrilic colloid
(C) Gold sol R. Collodion
(D) Cellulose nitrate in alcohol S. Lyophobic colloid
(a) A–R, B–S, C–Q, D–P
(b) A–P, B–Q, C–S, D–R
(c) A–R, B–S, C–P, D–Q
(d) A–P, B–Q, C–R, D–S
Answer
B