Please refer to Alcohols Phenols and Ethers HOTs Class 12 Chemistry provided below with Alcohols Phenols and Ethers. All HOTs for Class 12 Chemistry with answers provided below have been designed as per the latest syllabus and examination petter issued by CBSE, NCERT, KVS. Students of Standard 12 Chemistry should learn the solved HOTS for Class 12 Chemistry provided below to gain better marks in examinations.
Alcohols Phenols and Ethers Class 12 Chemistry HOTs
Question. n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent?
(a) PCl5
(b) Reduction
(c) Oxidation with potassium dichromate
(d) Ozonolysis
Answer
C
Question. Widespread deaths due to liquor poisoning occurs due to
(a) presence of carbonic acid in liquor
(b) presence of ethyl alcohol in liquor
(c) presence of methyl alcohol in liquor
(d) presence of lead compounds in liquor
Answer
C
Question. To distinguish between salicylic acid and phenol, one can use
(a) NaHCO3 solution
(b) 5% NaOH solution
(c) neutral FeCl3
(d) bromine water
Answer
A
Question. Acidity of phenol is due to
(a) hydrogen bonding
(b) phenolic group
(c) benzene ring
(d) resonance stabilisation of its anion
Answer
D
Question. The correct order of boiling points for primary (1º), secondary (2º) and tertiary alcohol (3º) is
(a) 1º > 2º > 3º
(b) 3º > 2º > 1º
(c) 2º > 1º > 3º
(d) 2º > 3º > 1º
Answer
A
Question. ‘Drinking alcohol’ is very harmful and it ruins the health.
‘Drinking alcohol’ stands for
(a) drinking methyl alcohol
(b) drinking ethyl alcohol
(c) drinking propyl alcohol
(d) drinking isopropyl alcohol
Answer
B
Question. Which of the following compounds is oxidised to prepare methyl ethyl ketone?
(a) 2-Propanol
(b) l-Butanol
(c) 2-Butanol
(d) t-Butyl alcohol
Answer
C
Question. Which of the following has lowest boiling point ?
(a) p-Nitrophenol
(b) m-Nitrophenol
(c) o-Nitrophenol
(d) Phenol
Answer
C
Question. The fermentation of starch to give alcohol occurs mainly with the help of
(a) O2
(b) air
(c) CO2
(d) enzymes
Answer
D
Question. Which one of the following alcohols is least soluble in water?
(a) CH3OH
(b) C3H7OH
(c) C4H9OH
(d) C10H21OH
Answer
D
Question. When phenol is treated with excess bromine water, it gives:
(a) m-Bromophenol
(b) o- and p-Bromophenol
(c) 2, 4-Dibromophenol
(d) 2, 4, 6-Tribromophenol
Answer
D
Question. Which of the following is correct ?
(a) On reduction of any aldehyde, secondary alcohol is formed
(b) Reaction of vegetable oil with H2SO4 gives glycerine
(c) Sucrose on reaction with NaCl gives invert sugar
(d) Alcoholic iodine gives iodoform with NaOH
Answer
D
Question. When ethyl alcohol reacts with acetic acid, the products formed are
(a) Sodium ethoxide + hydrogen
(b) Ethyl acetate + water
(c) Ethyl acetate + soap
(d) Ethyl alcohol + water
Answer
B
Question. Which of the following is not true in case of reaction with heated copper at 300°C?
(a) Phenol → Benzyl alcohol
(b) Secondary alcohol → Ketone
(c) Primary alcohol → Aldehyde
(d) Tertiary alcohol → Olefin
Answer
A
Question. Which statement is not correct about alcohol?
(a) Molecular weight of alcohol is higher than water
(b) Alcohol of less no. of carbon atoms is less soluble in water than alcohol of more no. of carbon atoms
(c) Alcohol evaporates quickly
(d) All of the above
Answer
B
Question. Which one of the following compounds has the most acidic nature?
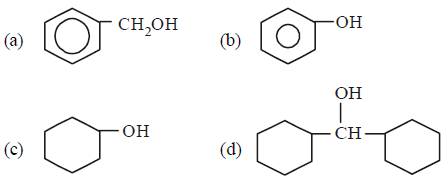
Answer
B
Question. In the reaction,

C is (where R = C6H5)
(a) NH3
(b) N2
(c) O2
(b) CO2
Answer
B
Question. The ionization constant of phenol is higher than that of ethanol because :
(a) phenoxide ion is bulkier than ethoxide
(b) phenoxide ion is stronger base than ethoxide
(c) phenoxide ion is stabilized through delocalization
(d) phenoxide ion is less stable than ethoxide
Answer
C
Question. Primary and secondary alcohols on action of reduced copper give
(a) Aldehydes and ketones respectively
(b) Ketones and aldehydes respectively
(c) Only aldehydes
(d) Only ketones
Answer
A
Question. Phenols do not react with one of the following :
(a) Alkali metals
(b) Sodium hydroxide
(c) Potassium hydroxide
(d) Sodium bi-carbonate
Answer
D
Question. HBr reacts fastest with
(a) 2-Mehtylpropan-1-ol
(b) 2-Methylpropene-2-ol
(c) propan-2-ol
(d) propan-1-ol
Answer
B
Question. The compound which reacts fastest with Lucas reagent at room temperature is
(a) Butan-1-ol
(b) Butan-2-ol
(c) 2-Methyl propan-1-ol
(d) 2-Methylpropan-2-ol
Answer
D
Question. Which of the following has strongest hydrogen bonding?
(a) Ethyl amine
(b) Ethanal
(c) Ethyl alcohol
(d) Diethyl ether
Answer
C
Question. Phenol is more acidic than alcohol because
(a) phenol is more stable than water
(b) phenol is aromatic and alcohol is aliphatic
(c) phenoxide ion is resonance stabilised
(d) None of these
Answer
C
Question. Oxygen atom in ether is
(a) very active
(b) replaceable
(c) comparatively inert
(d) active
Answer
C
Question. On distilling phenol with Zn dust, one gets :
(a) Toluene
(b) Benzaldehyde + ZnO
(c) ZnO + benzene
(d) Benzoic acid
Answer
C
Question. In the commercial manufacture of ethyl alcohol from starchy substances by fermentation method. Which enzymes slipwise complete the fermentation reaction
(a) Diastase, maltase and zymase
(b) Maltase, zymase and invertase
(c) Diastase, zymase and lactase
(d) Diastase, invertase and zymase
Answer
A
Question. Methanol and ethanol are miscible in water due to
(a) covalent character
(b) hydrogen bonding character
(c) oxygen bonding character
(d) None of these
Answer
B
Question. Dehydration of 2-butanol yields
(a) 1-butene
(b) 2-butene
(c) 2-butyne
(d) Both (a) and (b)
Answer
D
Question. The ether that undergoes electrophilic substitution reactions is
(a) CH3OC2H5
(b) C6H5OCH3
(c) CH3OCH3
(d) C2H5OC2H5
Answer
B
Question. Which of the following is most acidic?
(a) Benzyl alcohol
(b) Cyclohexanol
(c) Phenol
(d) m-Chlorophenol
Answer
D
Question. Diethyl ether on heating with conc. HI gives two moles of
(a) ethanol
(b) iodoform
(c) ethyl iodide
(d) methyl iodide
Answer
B
Question. Lucas test is done to differentiate between
(a) alcohol and ketone
(b) alcohol and aromatic ketones
(c) 1°, 2° and 3° alcohols
(d) None of these
Answer
C
Question. Commercially carboxylic acids are reduced to alcohols by converting them to the ______.
(a) esters
(b) aldehydes
(c) ketones
(d) amines
Answer
A
Question. Ethyl alcohol exhibits acidic character on reacting it with
(a) acetic acid
(b) sodium metal
(c) hydrogen chloride
(d) acidic K2Cr2O7
Answer
B
Question. An industrial method of preparation of methanol is :
(a) catalytic reduction of carbon monoxide in presence of ZnO–Cr2O3
(b) by reacting methane with steam at 900ºC with a nickel catalyst
(c) by reducing formaldehyde with lithium aluminium hydride
(d) by reacting formaldehyde with aqueous sodium hydroxide solution
Answer
A
Question. If ethanol dissolves in water, then which of the following would be observed
(a) absorption of heat and contraction in volume
(b) emission of heat and contraction in volume
(c) absorption of heat and increase in volume
(d) emission of heat and increase in volume
Answer
B
Question. In the following reaction,
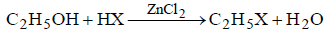
(a) CH2 = CH2
(b) CH3CH2OCH2CH3
(c) CH3CH2–HSO4
(d) (CH3CH2)2SO4
Answer
A
Question. Select the incorrect statement about the fermentation.
(a) When grapes are crushed, sugar and the enzyme come in contact and fermentation starts
(b) Fermentation takes place in anaerobic conditions
(c) Carbon monoxide is released during fermentation
(d) If air gets into fermentation mixture, the oxygen of air oxidises ethanol to ethanoic acid which in turn destroys the taste of alcoholic drinks
Answer
C
Question. Chemical name of salol is
(a) acetylsalicyclic acid
(b) sodium salicylate
(c) phenyl salicylate
(d) methyl salicylate
Answer
C