Please refer to Chemical Kinetics MCQ Questions Class 12 Chemistry below. These MCQ questions for Class 12 Chemistry with answers have been designed as per the latest NCERT, CBSE books and syllabus issued for the current academic year. These objective questions for Chemical Kinetics will help you to prepare for the exams and get more marks.
Chemical Kinetics MCQ Questions Class 12 Chemistry
Please see solved MCQ Questions for Chemical Kinetics in Class 12 Chemistry. All questions and answers have been prepared by expert faculty of standard 12 based on latest examination guidelines.
Question. What is the formula to find the value of t1/2 for a zero order reaction ?
(a) k/[R]0
(c) 2k/[R]0
(b) [R]0/2k
(d) 0.693/k
Answer
C
Question. CH3COOC2H5 + H2O →H+ CH3COOH + C2H5OH is an example of …… order.
(a) zero
(b) second
(c) third
(d) pseudo first order
Answer
D
Question. After how many seconds will the concentration of the reactant in a first order reaction be halved if the rate constant is 1.155 x 10-3 s-1?
(a) 600
(b) 100
(c) 60
(d) 10
Answer
A
Question. For a certain reaction the rate law is rate = k [C]3/2 . If the rate of the reaction is 0.020 mol-1 s-1 when [C] = 1.0 M, what is the rate when [C] = 0.60 M?
(a) 0.0093 mol-1 s-1
(b) 0.012 mol-1 s-1
(c) 0.033 mol-1 s-1
(d) 0.040 mol-1 s-1
Answer
A
Question. For a first order reaction, the half-life period is
(a) dependent on the square of the initial concentration
(b) dependent on first power of initial concentration
(c) dependent on the square root of initial concentration
(d) independent on initial concentration
Answer
D
Question. Give relation between halfreaction time (t1/2 ) and initial concentration ofreactant for (n — 1) order reaction.
(a) t1/2 ∝ [R]0
(b) t1/2 ∝ [R]02-n
(c) t1/2 ∝[R]0n+1
(d) t1/2 ∝ [R]0n-1
Answer
B
Question. For a reaction between gaseous compounds,
2A + B ➔ C + D
the reaction rate= k [A][B]. If the volume of the container is made 1/4 of the initial, then what will be the rate of reaction as compared to the initial rate?
(a) 16 times
(b) 4 times
(c) 1/8 times
(d) 1/16 times
Answer
A
Question. The plot between concentration versus time for a zero order reaction is represented by

Answer
D
Question. In a first order reaction, the concentration of the reactant decrease from 0.6 M to 0.3 Min 15 min. The time taken for the concentration to change from 0.1 M to 0.025 M in minutes is
(a) 1.2
(b) 12
(c) 30
(d) 3
Answer
C
Question. 2N2O5 ⇌ 4NO2 + O2,
If rate and rate constant for above reaction are 2.40 x 10-5 mol L-1 s-1 and 3 x 10-5 s-1 respectively, then calculate the concentration of N2O5.
(a) 1.4
(b) 1.2
(c) 0.04
(d) 0.8
Answer
D
Question. For a first order reaction, the initial concentration of a reactant is 0.05 M After 45 min itis decreased by 0.015 M Calculate halfreaction time (t1/2 )
(a) 88.84 min
(b) 25.90min
(c) 78.72min
(d) 77.20min
Answer
A
Question. In the reaction, P + Q → R + S the time taken for 75% reaction of Pis twice the time taken for 50% reaction of P. The concentration of Q. Q varies with reaction time as shown in the figure. The overall order of the reaction is
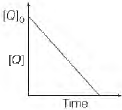
(a) 2
(b) 3
(c) 0
(d) 1
Answer
D
Question. Decay of 92U235 is …… order reaction.
( a) zero
(b) first
(c) second
(d) third
Answer
B
Question. For the non-stoichiometric reaction 2A + B → C + D, the following kinetic data were obtained in three separate experiments, all at 298 K
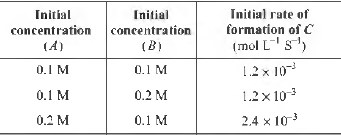
The rate law for the formation of C is
(a) dC / dt = k[A][B]
(b) dC / dt = k[A]2[B]
(c) dC / dt = k [A][B]2
(d) dC / dt = k[A]
Answer
D
Question. The conversion of A to B follows second order kinetics, doubling the concentration of A will increase the rate of formation of B by a factor of
(a) 4
(b) 2
(c) 1/4
(d) 1/2
Answer
A
Question. A(g) →Δ P(g) + Q(g) + R(g ‘), follows first order kinetics with a half-life of 69.3 s at 500°C. Starting from the gas ‘A’ enclosed in a container at 500° C and at a pressure of 0.4 atm, the total pressure of the system after 230 s will be
(a) 1.15 atm
(b) 1.32 atm
(c) 1.22 atm
(d) 1.12 atm
Answer
D
Question. The half-life of two samples are 0.1 and 0.8 s. Their respective concentration are 400 and 50 respectively. The order of the reaction is
(a) 0
(b) 2
(c) 1
(d) 4
Answer
B
Question. The half-life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is
(a) 0.5
(b) 1
(c) 2
(d) 0
Answer
C
Question. Fora first order reaction of k = 6.2 x 10-5 s-1 , t3/4 will be
(a) 4.65 X 10-5 s
(b) 2.24 X 10-4 s
(c) 2.24 x 10-5s
(d) 8.27x 10-5 s
Answer
B
Question. 75% of a first order reaction is completed in 30 min. What is the time required for 93.75% completion of the reaction (in minutes)?
(a) 45
(b) 120
(c) 90
(d) 60
Answer
D
Question. The half-life of two samples are 0.1 and 0.4 seconds. Their respective concentration are 200 and 50, respectively. What is the order ofreaction?
(a) 0
(b) 2
(c) 1
(d) 4
Answer
B
Question. In a reaction A + B ➔ C, the rate expression is R = k [A][ B]2. If the concentration of both the reactants is doubled at constant volume then the rate of the reaction will be
(a) eight times
(b) double
(c) quadmple
(d) tripleH
Answer
A
Question. The unit of second order reaction rate constant is
(a) L-1 mol s-1
(b) L-2 mol-2 s-1
(c) L mol-1 s-1
(d) s-1
Answer
C
Question. An organic compound undergoes first order decomposition. The time taken for its decomposition to 1/8 and 1/10 of its initial concentration are t1/8 and t1/10 , respectively. What is the value of [t1/8/t1/10] X 10 ?
(log10 2= 0.3)
(a) 2
(b) 3
(c) 5
(d) 9
Answer
D
Question. The following data were obtained during the first order decomposition of 2A(g) ➔ B(g)+C(s) at a constant volwne and at a particular temperature.

The rate constant in min-1 is
(a) 0.0693
(c) 6.93
(b) 69.3
(d) 6.93 x 10-4
Answer
A
Question. The time required for 100% completion of a zero order reaction is
(a) ak
(b) a / 2k
(c) a / k
(d) 2k / a
Answer
C
Question. Consider the following statements in respect of zero order reaction.
I. The rate of the reaction is independent of reactant concentration.
II. The rate of the reaction is independent of temperature.
III. The rate constant of the reaction is independent of temperature.
IV. The rate constant of the reaction is independent of reactant concentration.
Choose the correct statement/s.
(a) Only I
(b) I and II
(c) III and IV
(d) I and Ill
(e) I and IV
Answer
E
Question. For a first order reaction, (A) → products, the concentration of A changes from 0.1 M to 0.025 M in 40 min. The rate of reaction when the concentration of A is 0.01 Mis
(a) 1.73 x 10-5 M/min
(b) 3.47 X 10-4 M/min
(c) 3.47 x 10-5 M/min
(d) 1.73 x 10-4 M/min
Answer
B
Question. Consider the reaction,
Cl2 (aq) + H2S(aq) ➔ S(s) + 2H+ (aq) + 2Cl– (aq) The rate equation for this reaction is, rate= k [Cl2 ][H2SJ Which of these mechanisms is/are consistent with this rate equation?
I. Cl2 + H2S ➔ H+ + CI– + Cl+ + HS– (slow)
Cl+ + HS ➔ H+ + CI– + S(fast)
II. H2S ⇌ H+ + HS (fast equilibrium)
Cl2 + HS ➔ 2CI– + H+ + S(slow)
(a) Only II
(b) Both I and II
(c) Neither I nor II
(d) Only I
Answer
D
QuestionV. For a reaction between A and B, the initial rate ofreaction is measured for various initial concentrations of A andB. The data provided are

The overall order of the reaction is
(a) one
(b) two
(c) two and half
(d) three
Answer
A
Question. A+ B ➔ Product If concentration of A is doubled, rate increases 4 times. If concentrations of A and B both are doubled, rate increases 8 times. The differential rate equation of the reaction will be
(a) dC = kCA X CB
(b)dC = kC2A X C3B
(c) dC = kC2A X CB
(d) dC = kC2A X C2B
Answer
C
Question. For a first order reaction, the concentration changes from 0.8 to 0.4 in 15 min. The time taken for the concentration to change from 0.1 M to 0.025 M is
(a) 30 min
(b) 15 min
(c) 7.5 min
(d) 60 min
Answer
A
Question. For a zero order reaction, the plot of concentration of reactant versus time is (intercept refers to concentration axis)
(a) linear with positive slope and zero intercept
(b) linear with negative slope and zero intercept
(c) linear with negative slope and non-zero intercept
(d) linear with positive slope and non-zero intercept
(e) a curve asymptotic to concentration axis
Answer
C
Question. The concentration of R in the reaction R ➔ P was measured as a function of time and the following data is obtained
[R](molar) 1.0 0.75 0.40 0.10
t (min) 0.0 0.05 0.12 0.18
The order of the reaction is
(a) zero
(b) first
(c) second
(d) third
Answer
A
Question. For a first order reaction the ratio of times to complete 99 .9% and half of the reaction is
(a) 8
(b) 9
(c) 10
(d) 12
Answer
C
Question. Given the hypothetical reaction mechanism
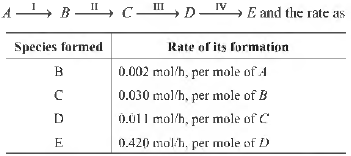
The rate determining step is
(a) step I
(b) step II
(c) step III
(d) step IV
Answer
A
Question. The half-life period of a first order reaction is I min 40 s. Calculate its rate constant.
(a) 6.93 x 10-3 min-1
(b) 6.93 x 10-3 s-1
(c) 6.93 X 10-3 s
(d) 6.93 X 103 s
Answer
B
Question. The time for half-life period of a certain reaction A ➔ products is 1 h. When the initial concentration of the reactant ‘A’, is 2.0 mol L-1 , how much time does it take for its concentration to come from 0.50 to 0.25 mol L-1 , if it is a zero order reaction ?
(a) 4 h
(b) 0.5 h
(c) 0.25 h
(d) 1 h
Answer
C
Question. For fourth order reaction, what is the unit of k?
(a) (mol / L)-3 s-1
(b) (mol/L)+3 s-1
(c) (mol/L)-3s
(d) (mol/L)-3
Answer
A
Question. For a reaction, A + 2B → C, rate is given by + d[C]/dt = k[A][B] , hence, the order of the reaction is
(a) 3
(b) 2
(c) 1
(d) 0
Answer
B
Question. For the second order reaction, A + B → Products. When a moles of A react with b moles of B, the rate equation is given by
k2t = 1 / (a – b) ln b(a – x) / a(b – x)
When a>> b, the rate expression becomes that of
(a) first order
(b) zero order
(c) unchanged, second order
(d) third order
Answer
A
Question. Ifaplot of log10 C versus t gives a straight line for a given reaction, then the reaction is
(a) zero order
(b) first order
(c) second order
(d) third order
Answer
B
Question. Under the same reaction conditions, initial concentration of 1.386 mo I dm-3 of a substance becomes half in 40 s and 20 s through first order and zero order kinetics respectively. Ratio(k1/k0) of the rate constants for first order ( k1 ) and zero ko order (k0 ) of the reaction is
(a) 0.5 mol-1 dm-3
(b) 1.0 mol dm-3
(c) 1.5 mol dm-3
(d) 2.0 mol-1 dm-3
Answer
A
Question. A first order reaction is 60% complete in 20 min. How long will the reaction take to be 84% complete?
(a) 68 min
(b) 40 min
(c) 76 min
(d) 54 min
Answer
B
Question. A hypothetical reaction, A2 + B2 → 2AB follows the mechanism as given below,
A2 ⇌ A + A (fast)
A + B2 → AB + B (slow)
A + B → AB (fast)
The order of the overall reaction is
(a) 2
(b) 1
(c) 1 1/2
(d) 0
Answer
C
Question. Consider the following statements, The rate law for the acid catalysed hydrolysis of an ester being given as Rate = k [H+ ][ester] = k’ [acid]. If the acid concentration is doubled at constant ester concentration
I. The second order rate constant, k is doubled.
II. The pseudo first order rate constant, k is doubled.
III. The rate of the reaction is doubled.
Which of the above statements are correct ?
(a) I and II
(b) II and III
(c) I and III
(d) I,II and III
Answer
B
Question. The order of a reaction with rate equal to kCA3/2 C8-1/2 is
(a) 1
(b) – 1/2
(c) – 3/2
(d) 2
Answer
A
Question. Consider a reaction; aG + bH ➔ products
When concentration of both the reactants G and H is doubled, the rate increases by eight times. However, when concentration ofG is doubled keeping the concentration of H fixed, the rate is doubled. The overall order of the reaction is,
(a) 0
(b) 1
(c) 2
(d) 3
Answer
D
Question. For the reaction, 2A + B → A2B, the rate law given is
(a) k[2A][B]
(c) k[A][B]3
(b) k[A]3[B]
(d) k[A]2[B]
Answer
D
Question. A first order reaction has a rate constant 1.15 x 10-3 s-1 . How long will 5g of this reactant take to reduce to 3g?
(a) 444 s
(b) 402 s
(c) 442 s
(d) None of these
Answer
A
Question. Which one of the following is a second order reaction ?
(a) H2 + Br2 → 2HBr
(b) NH4NO3 → N2 + 3H2O
(c) H2 + Cl2 →Sunlight 2HCI
(d) CH3COOCH3 + NaOH →
CH3COONa + CH3OH
Answer
D
Question. At 500 K, the half-life period of a gaseous reaction at an initial pressure of 80 kPa is 350 s. When the pressure is 40 kPa, the half-life period is 175 s. The order of the reaction is
(a) zero
(b) one
(c) two
(d) three
(e) half
Answer
A
Question. For the reaction, 2A + B ➔ C + D, the orderofreaction is
(a) one with respect to [B]
(b) two with respect to [A]
(c) three
(d) Cannot be predicted
Answer
D
Question. A reaction proceeds by first order, 75% of this reaction was completed in 32 min. The time required for 50% completion is
(a) 8 min
(b) 16 min
(c) 20 min
(d) 24 min
Answer
B
Question. The rate of the reaction A ➔ products, at the initial concentration of 3.24 x 10-2 M is nine times its rate at another initial concentration of 1.2 x 10-3 M. The order of the reaction is
(a) 1 / 2
(d) 3 / 4
(c) 3 / 2
(b) 2 / 3
(e) 1 / 3
Answer
D
Question. The half-life period of a first order chemical reaction is 6.93 min. The time required for the completion of 99% of the chemical reaction will be (log 2 = 0.301)
(a) 230.3 min
(b) 23.03 min
(c) 46.06 min
(d) 460.6 min
Answer
C
Question. The half-life period of a first order reaction is 69.3 s. What is the rate constant ?
(a) 0.01 s-1
(b) 0.1 s-1
(c) 1 s-1
(d) 10 s-1
Answer
A
Question. A reaction was observed for 15 days and the percentage of the reactant remaining after the days indicated was recorded in the following table.

Which one of the following best describes the order and the half-life of the reaction ?
Reaction order Half-life (days)
(a) First 2
(b) First 6
(c) Second 2
(d) Zero 6
(e) Third 2
Answer
C
Question. The concentration of a reactant X decreases from 0.1 M to 0.005 M in 40 min. If the reaction follows first order kinetics, the rate of the reaction when the concentration of Xis 0.01 M will be
(a) 1.73 x 10-4 M min-1
(b) 3.47 X 10-4 M min-1
(c) 3.47 x 10-5 M min-1
(d) 7.5 x 10-4 M min-1
Answer
D
Question. A reaction involving A, Band C as reactants is found to obey the rate law, rate = k[A]x[B]Y[C]z . When the concentrations of A,B and Care doubled separately, the rate is also found to increase two, zero and four times respectively. The overall order of the reaction is
(a) 1
(b) 2
(c) 3
(d) 4
Answer
C
Question. For a zero order reaction
(a) t1/2 ∝ R0
(b) t1/2 ∝ 1/ R0
(c) t1/2 ∝ R02
(d) t1/2 ∝ 1/ R02
Answer
A
Question. In the first order reaction, 75% of the reactant gets disappeared in 1.386 h. The rate constant of the reaction is
(a) 3.0 x 10-3 s-1
(b) 2.8 x 10-4 s-1
(c) 17.2 x 10-3 s-1
(d) 1.8 x 10-3 s-1
Answer
B
Question. Consider the reaction, 2A + B ➔ products When concentration of B alone was doubled, the half-life did not change. When the concentration of A alone was doubled, the rate increased by two times. The unit of rate constant for this reaction is
(a) L mol-1 s-1
(b) no unit
(c) mol L-1 s-1
(d) s-1
Answer
A
Question. The rate of first order reaction is 1.5 x 10-2 mol L-1 min-1 at 0.5 M concentration of the reactant. The half-life of the reaction is
(a) 0.383 min
(b) 23.1 min
(c) 8.73 min
(d) 7.53 min
Answer
B
Question. The following data are for the decomposition of ammonium nitrite in aqueous solution.
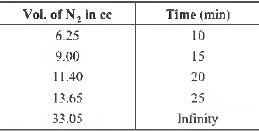
The order of reaction is
(a) zero
(b) one
(c) two
(d) three
Answer
B
Question. For a reaction, the rate constant is 2.34s-1 . The half-life period for the reaction is
(a) 0.30 s
(b) 0.60 s
(c) 3.3 s
(d) data is insufficient
Answer
A
Question. The rate law for a reaction between the substances A and Bis given by rate = k[A]n[B]m. On doubling the concentration of A and halving the concentration of B, the ratio of the new rate to the earlier rate of the reaction will be as
(a) 1/2m+n
(b) (m + n)
(c) (n – m)
(d) 2(n – m)
Answer
D
Question. For the reaction system,
2NO(g) + O2(g) → 2NO2(g)
volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with respect to NO; the rate of reaction will
(a) diminish to one-fourth of its initial value
(b) diminish to one-eighth of its initial value
(c) increase to eight times of its initial value
(d) increase to four times of its initial value
Answer
C
Question. The t1/2 of the first order reaction is
(a) dependent of initial concentration
(b) directly proportional to initial concentration
(c) indirectly proportional to initial concentration
(d) independent of initial concentration
Answer
D
Question. Consider an endothermic reaction X → Y with the activation energies Eb and Ef for the backward and forward reactions, respectively. In general
(a) there is no definite relation between Eb and Ef
(b) Eb = Ef
(c) Eb > Ef
(d) Eb < Ef
Answer
D
Question. In a reaction, the threshold energy is equal to
(a) activation energy + normal energy of reactants
(b) activation energy – normal energy of reactants
(c) normal energy of reactants – activation energy
(d) average kinetic energy of molecules of reactants
Answer
A
Question. The energies of activation for forward and reverse reactions for A2 + B2 ⇌ 2AB are 180 kJ mol–1 and 200 kJ mol–1 respectively. The presence of a catalyst lowers the activation
energy of both (forward and reverse) reactions by 100 kJ mol–1. The enthalpy change of the reaction (A2 + B2 → 2AB) in the presence of a catalyst will be (in kJ mol–1)
(a) 20
(b) 300
(c) 120
(d) 280
Answer
A
Question. The temperature dependence of rate constant (k) of a chemical reaction is written in terms of Arrhenius equation,
k = A . e-Ea Activation energy (Ea) of the reaction can be calculated by plotting
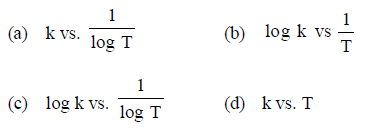
Answer
B
Question. According to which theory activation energy and proper orientation of the molecules together determine the criteria for an effective collision ?
(a) Arrhenius theory
(b) Activated complex theory
(c) Collision theory
(d) Both (a) and (c)
Answer
C
Question. Activation energy of a chemical reaction can be determined by ___________
(a) determining the rate constant at standard temperature.
(b) determining the rate constants at two temperatures.
(c) determining probability of collision.
(d) using catalyst.
Answer
B
Question. For the exothermic reaction A + B → C + D,ΔH is the heat of reaction and Ea is the energy of activation. The energy of activation for the formation of A + B will be
(a) Ea
(b) ΔH
(c) Ea + ΔH
(d) ΔH – Ea
Answer
C
Question. Activation energy of the reaction is
(a) the energy released during the reaction
(b) the energy evolved when activated complex is formed
(c) minimum amount of energy needed to overcome the potential barrier
(d) the energy needed to form one mole of the product
Answer
C
Question. A catalyst increases rate of reaction by
(a) decreasing enthalpy
(b) decreasing internal energy
(c) decreasing activation energy
(d) increasing activation energy
Answer
C
Question. Consider Fig. and mark the correct option.

(a) Activation energy of forward reaction is E1 + E2 and product is less stable than reactant.
(b) Activation energy of forward reaction is E1 + E2 and product is more stable than reactant.
(c) Activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than product.
(d) Activation energy of backward reaction is E1 and product is more stable than reactant.
Answer
A
Question. In most cases, for a rise of 10K temperature the rate constant is doubled to tribled. This is due to the reason that
(a) collision frequency increases by a factor of 2 to 3.
(b) fraction of molecules possessing threshold energy increases by a factor of 2 to 3
(c) Activation energy is lowered by a factor of 2 to 3.
(d) none of these
Answer
B
Question. 1/[A]2 versus time is a straight line. Order of reaction is
(a) first
(b) second
(c) zero
(d) third
Answer
D



