VBQs Chemical Reactions and Equations Class 10 Science with solutions has been provided below for standard students. We have provided chapter wise VBQ for Class 10 Science with solutions. The following Chemical Reactions and Equations Class 10 Science value based questions with answers will come in your exams. Students should understand the concepts and learn the solved cased based VBQs provided below. This will help you to get better marks in class 10 examinations.
Chemical Reactions and Equations VBQs Class 10 Science
Question. In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
(a) 2H2(l) + O2(l) → 2H2O(g)
(b) 2H2(g) + O2(l) → 2H2O(l)
(c) 2H2(g) + O2(g) → 2H2O(l)
(d) 2H2(g) + O2(g) → 2H2O(g)
Answer
C
Question. Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
(a) (i) only
(b) (ii) only
(c) (iv) only
(d) (ii) and (iv)
Answer
D
Question. Which of the following conditions is necessary for a chemical reaction?
(a) It must be accompanied with change in temperature and pressure.
(b) At least one of the reactants must be in a fixed quantity.
(c) It must follow the law of conservation of mass.
(d) All of the above.
Answer
C
Question. Three beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It w as observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the following statement(s) is (are) correct?
(i) In beakers A and B, exothermic process has occurred.
(ii) In beakers A and B, endothermic process has occurred.
(iii) In beaker C, exothermic process has occurred.
(iv) In beaker C, endothermic process has occurred.
(a) (i) only
(b) (ii) only
(c) (i) and (iv)
(d) (ii) and (iii)
Answer
C
Question. Which of the following reactions is an endothermic reaction?
(a) Burning of coal.
(b) Decomposition of vegetable matter into compost.
(c) Process of respiration.
(d) Decomposition of calcium carbonate to form quick lime and carbon dioxide.
Answer
D
Question. Which among the following is (are) double displacement reaction(s)?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 → CO2 + 2H2O U
(a) (i) and (iv)
(b) (ii) only
(c) (i) and (ii)
(d) (iii) and (iv)
Answer
B
Question. There is an equation ‘X’, which contains equal number of atoms of each element on both the sides. What is ‘X’?
(a) A balanced equation
(b) An unbalanced equation
(c) A chemical equation
(d) All of the above
Answer
A
Question. Which among the following is not a physical change ?
(a) Evaporation of petrol
(b) Burning of liquified petroleum gas (LPG)
(c) Heating of an iron rod to red hot.
(d) Sublimation of solid ammonium chloride
Answer
B
Question. The reaction in which a substance or substances undergo change to produce new substances with new properties is called
(a) A biochemical reaction
(b) A nuclear reaction
(c) A physical reaction
(d) A chemical reaction
Answer
D
Question. In the given equation, what does ‘X’ stand for ?
(2)Al + (X)H2SO4 → Al2(SO4)3 + (3)H2
(a) 2
(b) 3
(c) 1
(d) 5
Answer
B
Question. Which of the following is not a physical change?
(a) Boiling of water to give water vapour
(b) Melting of ice to give water
(c) Dissolution of salt in water
(d) Combustion of liquified petroleum gas (LPG)
Answer
D
Question. The following reaction is an example of a 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
(i) Displacement reaction
(ii) Combination reaction
(iii) Redox reaction
(iv) Neutralisation reaction
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (iii) and (iv)
Answer
B
Question. A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?
(a) KMnO4 is an oxidising agent, it oxidises FeSO4.
(b) FeSO4 acts as an oxidising agent and oxidises KMnO4.
(c) The colour disappears due to dilution; no reaction is involved.
(d) KMnO4 is an unstable compound and decomposes in presence of FeSO4 to a colourless compound.
Answer
A
Question. Which one of the following processes involve chemical reactions?
(a) Storing of oxygen gas under pressure in a gas cylinder
(b) Liquification of air
(c) Keeping petrol in a china dish in the open
(d) Heating copper wire in presence of air at high temperature
Answer
D
Assertion And Reason Based MCQs :
Directions : In the following questions, A statement of Assertion (a) is followed by a statement of Reason (R). Mark the correct choice as.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is NOT the correct explanation of A.
(c) A is true but R is false.
(d) A is false and R is true.
Question. Assertion (A): Carbon dioxide turns lime water milky.
Reason (R): Carbon dioxide sullies the water.
Answer
C
Question. Assertion (A): A chemical reaction becomes faster at higher temperatures.
Reason (R): At higher temperatures, molecular motion becomes more rapid.
Answer
A
Question. Assertion (a): After white washing the walls, a shiny white finish on walls is obtained after two to three days.
Reason (R): Calcium oxide reacts with carbon dioxide to form calcium hydrogen carbonate which gives shiny white finish.
Answer
C
Question. Assertion (A): Burning of candle is a physical change.
Reason (R): In physical change, no new substance is formed.
Answer
D
Question. Assertion (A): Sodium metal is stored under kerosene.
Reason (R): Metallic sodium melts when exposed to air.
Answer
C
Question. Assertion (A): To dilute sulphuric acid, acid is added to water and not water to acid.
Reason (R): Specific heat of water is quite large.
Answer
A
Question. Assertion: In the reaction : MnO2 + 4HCl → MnCl2 + 2H2O + Cl2 HCl is getting oxidized while MnO2 is getting reduced.
Reason: The process in which oxygen is added to a substance is called oxidation.
Answer
A
Question. Assertion (A): Chips manufacturers usually flush bags of chips with gas such as nitrogen.
Reason (R): Nitrogen gas prevents the oil and fats of the chips from being oxidized.
Answer
A
Case Based Question :
I. Read the following and answer any four questions from Marble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from handheld sculptures to massive pillars and buildings.

Question. Gas A, obtained above is a reactant for a very important biochemical process which occurs in the presence of sunlight. Identify the name of the process –
(a) Respiration
(b) Photosynthesis
(c) Transpiration
(d) Photolysis
Answer
B
Question. Marble statues are corroded or stained rain water.
Identify the main reason.
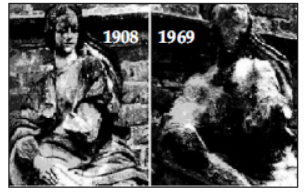
(a) decomposition of calcium carbonate to calcium oxide
(b) polluted water is basic in nature hence it reacts with calcium carbonate
(c) polluted water is acidic in nature hence it reacts with calcium carbonate
(d) calcium carbonate dissolves in water to give calcium hydroxide.
Answer
B
Question. Calcium oxide can be reduced to calcium, by heating with sodium metal. Which compound would act as an oxidizing agent in the above process?
(a) sodium
(b) sodium oxide
(c) calcium
(d) calcium oxide
Answer
D
Question. The substance not likely to contain CaCO3 is
(a) Dolomite
(b) A marble statue
(c) Calcined gypsum
(d) Sea shells.
Answer
C
Question. A student added 10g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 minutes and plotted against time, in a graph as shown below. During which time interval did maximum decomposition took place?
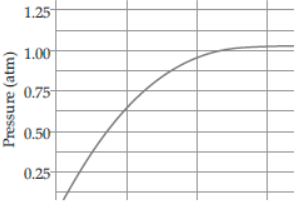
(a) 15-20 min
(b) 10-15 min
(c) 5-10 min
(d) 0-5 min
Answer
D
II. Read the following and answer any four questions from Chemistry in Automobiles: For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car,the distributor and battery provide this starting energy by creating an electrical “spark”,which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air:
2C8H18(l) + 25O2(g) ¾® 16 ‘X’ + Y
Question. ‘A student while walking on the road observed that a cloud of black smoke belched out from the exhaust stack of moving trucks on the road.’ Choose the correct reason for the production of black smoke:
(a) Limited supply of air leads to incomplete combustion of fuel.
(b) Rich supply of air leads to complete combustion of fuel.
(c) Rich supply of air leads to a combination reaction.
(d) Limited supply of air leads to complete combustion of fuel.
Answer
A
Question. ‘Although nitrogen is the most abundant gas in the atmosphere, it does not combustion’. Identify the correct reason for this statement.
(a) Nitrogen is a reactive gas
(b) Nitrogen is an inert gas
(c) Nitrogen is an explosive gas
(d) Only hydrocarbons can take part in combustion
Answer
B
Question. Which of the following are the products obtained from the reaction mentioned in the above case? Product ‘X’ Product ‘Y’
(a) CO2 H2O2
(b) H2O CO
(c) CH3OH H2O
(d) CO2 H2O
Answer
D
Question. Identify the types of chemical reaction occurring during the combustion of fuel:
(a) Oxidation & Endothermic reaction
(b) Decomposition & Exothermic reaction
(c) Oxidation & Exothermic reaction
(d) Combination & Endothermic reaction
Answer
C
Question. On the basis of evolution/absorption of energy, which of the following processes are similar to combustion of fuel?
(i) Photosynthesis in plants
(ii) Respiration in the human body
(iii) Decomposition of vegetable matter
(iv) Decomposition of ferrous sulphate.
(a) (ii) & (iii)
(b) (i) & (ii)
(c) (iii) & (iv)
(d) (ii) & (i)
Answer
A
III. Read the given passage and answer any four questions from The physical states of the reactants and products can be represented by using the symbols (s) for solids, (l) for liquids, (g) for gases and (aq) for aqueous solution along with their respective formulae. The word aqueous is written if the reactant or product is present as a solution in water. Precipitate can also be represented by using an arrow pointing downwards (↓) instead of using symbol (s).
In the same way, the gaseous state of an evolved gas can be represented by using an arrow pointing upward direction (↑) instead of using symbol (g). The specific condition of the reaction like temperature, pressure, catalyst etc. is written above or below the arrow in the chemical equation.
Question. If the reactant or product is present as a solution of water , it is represented as:
(a) (s)
(b) (l)
(c) (aq)
(d) ↓
Answer
C
Question. Which of the following reaction is balanced?
(a) NaCl + 2H2O → 2NaOH + 2Cl2 + H2
(b) 2NaCl + H2O → 2NaOH + 2Cl2 + H2
(c) 2NaCl + 2H2O → 2NaOH + Cl2 + H2
(d) 2NaCl + 2H2O → NaOH + Cl2 + H2
Answer
C
Question. Which of the following reaction is balanced?
(a) Mg (aq) + H2SO4 (aq) → MgSO4 (aq) + H2 ↑
(b) Mg (s) + H2SO4 (aq) → MgSO4 (aq) + H2 ↑
(c) Mg (s) + H2SO4 (l) → MgSO4 (l) + H2(g)
(d) Mg (s) + H2SO4 (l) → MgSO4 (s) + H2
Answer
B
Question. The correct way to represent the evolution of gas, is to use which of the following symbol:
(a) ↓
(b) →
(c) ↑
(d) (g)
Answer
C
Question. Complete the missing variable given as X and Y in the following reaction:
2Na (s) + 2H2O (l) → 2NaOH (X) + H2 (Y)
(a) (aq) and (g)
(b) (s) and (g)
(c) (g) and (l)
(d) (g) and (aq)
Answer
A
IV. Read the given passage and answer any four questions from In the following chemical reaction ‘‘zinc oxide reacts with carbon to produce zinc metal and carbon monoxide.’’
ZnO + C ® Zn + CO
Question. The reduction reaction involves:
(a) gain of electrons
(b) loss of electrons
(c) increase in oxidation state
(d) addition of oxygen
Answer
A
Question. Which of the following is the effect of oxidation reaction in everyday life:
(a) Precipitation
(b) Fermentation
(c) Corrosion
(d) Hydrogenation of oil
Answer
C
Question. The reactions used in black and white photography:
(a) Decomposition of silver bromide
(b) Decomposition of silver chloride
(c) Both
(d) None of the above
Answer
C
Question. Name the substance getting oxidised and reduced in the above reaction:
(a) C and ZnO
(b) Zn and C
(c) ZnO and CO
(d) CO and ZnO
Answer
A
Question. Name the type of reaction:
(a) oxidation reaction
(b) reduction reaction
(c) redox reaction
(d) decomposition reaction
Answer
C
V. P, Q and R are three elements which undergo chemical reactions according to the following equations. Answer any four question from
(i) P2O3 + 2Q ® Q2O3 + 2P
(ii) 3RSO4 + 2Q ® Q2(SO4)3 + 3R
(iii) 3RO + 2P ® P2O3 + 3R
Question. 3RSO4 + 2Q ® Q2(SO4)3 + 3R The given reaction shows:
(a) Q is more reactive than
(b) Q is less reactive than
(c) Q and R are equally reactive
(d) none of the above
Answer
A
Question. Choose the correct statement:
(a) Zinc and lead are more reactive elements than copper.
(b) Zinc and lead are less reactive elements than copper.
(c) Zinc and copper are more reactive elements than lead.
(d) Copper and lead are more reactive elements than zinc.
Answer
A
Question. Na2SO4 (aq) + BaCl2(aq) ® BaSO4 (s) + 2NaCl(aq) The above reaction is an example of:
(a) Double displacement reaction.
(b) Displacement reaction.
(c) Can be both.
(d) None of the above.
Answer
A
Question. The most reactive and the least reactive elements are:
(a) Q and P
(b) Q and R
(c) R and Q
(d) R and P
Answer
B
Question. The type of reaction is :
(a) Displacement reaction
(b) Combination reaction
(c) Neutralisation reaction
(d) Substitution reaction
Answer
A
VI. The following diagram displays a chemical reaction. Observe carefully and answer any four questions from
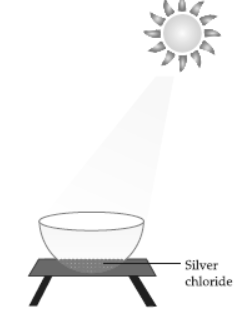
Question. The correct balanced chemical equation involves:
(a) 2AgCl (s) sunlight → 2Ag (s) + Cl2 (g)
(b) Ag + Cl → AgCl
(c) AgCl2 → Ag2 + Cl2
(d) AgCl sunlight → 2Ag + Cl2
Answer
A
Question. When decomposition is carried out by heating, it is called as:
(a) Heat decomposition
(b) Photolytic decomposition
(c) Electrolytic decomposition
(d) Thermal decomposition
Answer
D
Question. The other silver salt which behaves like silver chloride in sunlight is:
(a) silver hydride
(b) silver bromide
(c) silver iodide
(d) silver nitrite
Answer
B
Question. The type of chemical reaction that will take place is
(a) Photochemical decomposition
(b) Displacement reaction
(c) Reduction reaction
(d) Combination reaction
Answer
A
Question. What colour change is observed in silver chloride?
(a) Silver chloride turns white.
(b) Silver chloride turns brown.
(c) Silver chloride shows no colour change.
(d) White silver chloride changes to grey.
Answer
A