Students should refer to Worksheets Class 12 Chemistry Biomolecules Chapter 14 provided below with important questions and answers. These important questions with solutions for Chapter 14 Biomolecules have been prepared by expert teachers for Class 12 Chemistry based on the expected pattern of questions in the Class 12 exams. We have provided Worksheets for Class 12 Chemistry for all chapters on our website. You should carefully learn all the important examinations questions provided below as they will help you to get better marks in your class tests and exams.
Biomolecules Worksheet Class 12 Chemistry
Multiple Choice Questions (MCQs)
Question. Which base is present in RNA but not in DNA?
(a) Uracil
(b) Cytosine
(c) Guanine
(d) Thymine
Answer
D
Question. The functional group which is found in amino acid is
(a) – COOH
(b) – NH2
(c) – CH3
(d) both (a) and (b)
Answer
D
Question. Antibodies are –
(a) carbohydrates
(b) Proteins
(c) Lipids
(d) Enzymes
Answer
B
Question. ADP and ATP differ in the number of
(a) adenine base
(b) ribose units
(c) phosphate units
(d) nitrogen atoms
Answer
C
Question. Which of the following properties of glucose can be explained only by its cyclic structure
(a) Glucose forms pentaacetate
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidized by nitric acid to gluconic acid.
Answer
C
Question. In both DNA and RNA, heterocyclic base and phospho diester linkages are at –
a. C5′ and C2′ respectively of the sugar molecule
b. C2′ and C5′ respectively of the sugar molecule
c. C1′ and C5′ respectively of the sugar molecule
d. C5′ and C1′ respectively of the sugar molecule
Answer
C
Question. Which statement is incorrect about peptide bond?
(a) N bond length in proteins is longer than usual bond length of C – N bond
(b) Spectroscopic analysis shows planar structure of – CO – NH – group
(c) C – N bond length in proteins is smaller than usual bond length of C – N bond
(d) None of the above
Answer
A
Question. Protein is a polymer made of
(a) carbohydrates
(b) amino acids
(c) nucleic acids
(d) carboxylic acids
Answer
B
Question. Which of the following statements is not true about glucose ?
(a) It is an aldohexose
(b) On heating with HI it forms n- hexane
(c) It is present in furanose form
(d) It does not give 2,4-DNP test.
Answer
C
Question. Nucleic acids are the polymers of
(a) Nucleosides
(b) Nucleotides
(c) Bases
(d) Sugars
Answer
B
Question. Amylopectin is a polymer of
(a) b-D-glucose
(b) a-D-glucose
(c) b-D-frutose
(d) a-D-fructose
Answer
B
Question. Distinction between glucose and fructose can be done by
(a) Benedict’s solution
(b) Tollen’s reagent
(c) Selivanoff’s reagent
(d) Fehling solution
Answer
C
Question. DNA and RNA differ in
(a) Sugar
(b) Purines
(c) Pyrimidines
(d) Both (a) and (c)
Answer
D
Question. Which does not show mutarotation?
(a) Glucose
(b) Maltose
(c) Fructose
(d) Sucrose
Answer
D
Question. The sugar present in milk is
(a) Sucrose
(b) Maltose
(c) Glucose
(d) Lactose
Answer
D
Question. a-D (+) glucose and b-D (+) – glucose are
(a) Enantiomers
(b) Geometrical isomers
(c) Anomers
(d) Epimers
Answer
C
Question. The reagent used for obtaining osazone derivative of fructose is
(a) NH2OH
(b) NH2—NH2
(c) NH2—NHC6H5
(d) 2, 4—DNP
Answer
C
Question. The vitamin present in oils and fats are
(a) A and D
(b) B and C
(c) A and B
(d) A and C
Answer
A
Question. The disease resulting from the intake of amino acid deficient diet is
(a) Kwasiorkar
(b) Pernicicres anaemia
(c) PEM
(d) Haemophilio
Answer
A
Question. Keratin present in hair is an example of
(a) Fibrous protein
(b) Globular protein
(c) Conjugated protein
(d) Derived protein
Answer
A
Question. Hydrolysis of sucrose gives
(a) Glucose only
(b) Glucose + fructose
(c) Glucose and galactose
(d) Maltose
Answer
B
Assertion-Reason Question
DIRECTION: Mark the option which is most suitable:
(a) Assertion and Reason both are correct statements and Reason is correct explanation for Assertion.
(b) Assertion and Reason both are correct statements but Reason is not correct explanation for Assertion.
(c) Assertion is correct statement but reason is false statement.
(d) Assertion is false statement but reason is correct statement.
Question. Assertion: Glycine exists as Zwitter ion but oand p-aminobenzoic acids do not.
Reason: Due to the presence of –NH2 and –COOH group within the same molecule, they neutralise each other and hence, a-amino acids exist as dipolar ions or zwitter ions.
Answer
B
Question. Assertion: Fructose reduces Fehling’s solution and Tollens’ reagent.
Reason: Fructose does not contain any aldehyde group.
Answer
B
Question. Assertion: a-Amino acids exits as dipolar ions or zwitter ions.
Reason: a-Amino acids are the building blocks of proteins.
Answer
B
Question. Assertion: Glucose when treated with CH3OH in presence of dry HCl gas gives a-and b-methyl glucosides.
Reason: Glucose reacts with phenyl hydrazine to form crystalline osazone.
Answer
B
Question. Assertion: Uracil occurs in DNA.
Reason: DNA undergoes replication.
Answer
D
Question. Assertion: DNA undergoes replication.
Reason: DNA contains cytosine and thymine as pyrimidine bases.
Answer
B
Question. Assertion: Glucose and fructose are reducing sugars.
Reason: Glucose and fructose contain a free aldehydic and ketonic group adjacent to a > CHOH group respectively.
Answer
A
Question. Assertion: b-pleated sheet structure of protein shows maximum extension.
Reason: Intermolecular hydrogen bonding is present in them.
Answer
B
Question. Assertion: Alpha (a) amino acids exist as internal salt in solution as they have amino and carboxylic acid groups in near vicinity.
Reason: H+ ion given by carboxylic group (–COOH) is captured by amino group (–NH2) having lone pair of electrons.
Answer
A
Question. Assertion: Haemoglobin is a globular protein.
Reason: Globular proteins are insoluble in water.
Answer
C
A statement of assertion is followed by a statement of reason. Mark the correct choice from the options given below:
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Question. Assertion : Purine bases present in DNA are adenine and guanine.
Reason : The base thymine is present in RNA while base uracil is present in DNA.
Answer
C
Question. Assertion : D – glucose is dextrorotatory whereas L – glucose is levorotatory.
Reason : D – compounds are always dextro and L – compounds are always laevo.
Answer
B
Question. Assertion : Keratin is a globular protein.
Reason : Globular shape is due to secondary structure of proteins.
Answer
C
Question. Assertion : The two strands of DNA are complementary.
Reason : Cytosine always pairs with guanine and thymine pairs with adenine.
Answer
A
Question. Assertion : α – Amino acids are the building blocks of proteins.
Reason : Natural amino acids are mostly α – amino acids.
Answer
B
Case Based Questions
1. Pentose and hexose undergo intramolecular hemiacetal or hemiketal formation due to combination of the –OH group with the carbonyl group. The actual structure is either of five or six membered ring containing an oxygen atom. In the free state all pentoses and hexoses exits in pyranose form (resembling pyran). However, in the combined state some of them exits as five membered cyclic structures, called furanose (resembing furan).
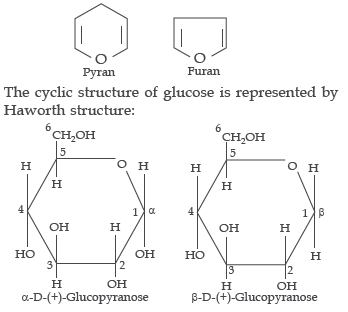
α and β-D-glucose have different configuration at anomeric (C-1) carbon atom, hence are called anomers and the C-1 carbon atom is called anomeric carbon (glycosidic carbon).
The six membered cyclic structure of glucose is
called pyranose structure.
Answer the following MCQs by choosing the most appropriate options:
Question. What percentage of b-D–(+) glucopyranose is found at equilibrium in the aqueous solution?
(a) 50%
(b) ≈ 100%
(c) 36%
(d) 64%
Answer
D
Question. The following carbohydrate is
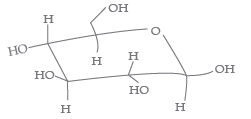
(a) a ketohexose
(b) an aldohexose
(c) an a-furanose
(d) an a-pyranose.
Answer
B
Question. In the following structure, anomeric carbon is

(a) C–1
(b) C–2
(c) C–3
(d) C–4 Ans.
Answer
A
Question. a-D(+)-glucose and b-D(+)–glucose are
(a) enantiomers
(b) conformers
(c) epimers
(d) anomers
Answer
D
Question. The term anomers of glucose refers to
(a) isomers of glucose that differ in configurations at carbons one and four (C–1 and C–4)
(b) a mixture of (D)-glucose and (L)-glucose
(c) enantiomers of glucose
(d) isomers of glucose that differ in configuration at carbon one (C–1).
Answer
D
2. Carbohydrates can exist in either of two conformations, as determined by the orientation of the hydroxyl group about the asymmetric carbon farthest from the carbonyl.

By convention, a monosaccharide is said to have D-configuration if the hydroxyl group attached to the asymmetric carbon atom adjacent to the –CH2OH group is on the right hand side irrespective of the positions of the other hydroxyl groups. On the other hand, the molecule is assigned L-configuration if the –OH group attached to the carbon adjacent to the –CH2OH group is on the left side.
Answer the following MCQs by choosing the most appropriate options:
Question. The two functional groups present in a typical carbohydrate are
(a) –OH and –COOH
(b) –CHO and –COOH
(c) >C = O and –OH
(d) –OH and –CHO
Answer
C
Question. Which of the following monosaccharides, is the majority found in the human body?
(a) D-type
(b) L-type
(c) Both of these
(d) None of these
Answer
A
Question. D-Glyceraldehyde and L-Glyceraldehyde are
(a) epimers
(b) enantiomers
(c) anomers
(d) conformational diasteriomers
Answer
B
Question. Monosaccharides contain
(a) always six carbon atoms
(b) always five carbon atoms
(c) always four carbon atoms
(d) may contain 3 to 7 carbon atoms
Answer
D
Question. The correct corresponding order of names of four aldoses with configuration given below respectively, is
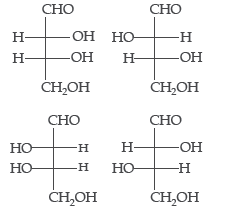
(a) L-erythrose, L-threose, L-erythrose, D-threose
(b) D-threose, D-erythrose, L-threose, D-erythrose
(c) L-erythrose, L-threose, D-erythrose, D-threose
(d) D-erythrose, D-threose, L-erythrose, D-threose
Answer
D
3. Carbohydrates are polyhydroxy aldehydes and ketones and those compounds which on hydrolysis give such compounds are also carbohydrates. The carbohydrates which are not hydrolysed are called monosaccharides. Monosaccharides with aldehydic group are called aldose and those which free ketonic groups are called ketose. Carbohydrates are optically active. Number of optical isomers = 2n
…[where n = number of asymmetric carbons Carbohydrates are mainly synthesised by plants during photosynthesis. The monosaccharides give the characteristic reactions of alcohols and carbonyl group (aldenydes and ketones). It has been found that these monosaccharides exist in the form of cyclic structures. In cyclization the –OH groups (generally C5 or C4 in aldohexoses and C5 or C6 in ketohexoses) combine with the aldehyde or keto group. As a result, cyclic structure of five or six membered rings containing one oxygen atom are formed, e.g., glucose forms a ring structure. Glucose contains one aldehyde group, one 1º alcholic group and four 2º alcoholic group in its open chain structure.
Answer the following MCQs by choosing the most appropriate options:
Question. First member of ketos sugar is
(a) ketotriose
(b) ketotetrose
(c) ketopentose
(d) ketohexose
Answer
A
Question. In
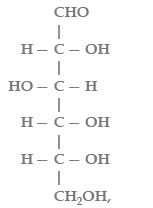
the number of optical isomers will be
(a) 16
(b) 8
(c) 32
(d) 4
Answer
A
Question. Some statements are given below:
I. Glucose is aldohexose.
II. Naturally occurring glucose is dextrorotatory.
III. Glucose contains three chiral centres.
IV. Glucose contains one 1º alcoholic group and four 2º alcoholic groups. Among the above, correct statements are
(a) I and II only
(b) III and IV only
(c) I, II and IV only
(d) I, II, III and IV
Answer
C
Question. Which of the following reactions of glucose can be explained only by its cyclic structure?
(a) Glucose forms cyanohydrin with HCN.
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidised by nitric acid to gluconic acid.
Answer
C
Question. Two hexoses form the same osazone, find the correct statement about these hexoses.
(a) Both of them must be aldoses.
(b) They are epimers at C-3
(c) The carbon atoms 1 and 2 in both have the same configuration.
(d) The carbon atoms 3, 4 and 5 in both have the same configuration.
Answer
D
4. When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein. The denaturation causes changed in secondary and tertiary structures but primary structures remains intact. Examples of denaturation of protein are coagulation of egg white on boiling, curdling of milk, formation of cheese when an acid is added to milk.
Answer the following MCQs by choosing the most appropriate options:
Question. Which structure (s) of proteins remain(s) intact during denaturation process?
(a) Both secondary and tertiary structure
(b) Primary structure only
(c) Secondary structure only
(d) Tertiary structure only
Answer
B
Question. Mark the wrong statement about denaturation of proteins.
(a) The primary structure of the protein does not change.
(b) Globular proteins are converted into fibrous proteins.
(c) Fibrous proteins are converted into globular proteins.
(d) The biological activity of the protein is destroyed.
Answer
C
Question. Secondary structured of protein refers to
(a) mainly denatured proteins and structure of prosthetic groups.
(b) three-dimensional structure, especially the bond between amino acid residues that are distant from each other in the polypeptide chain.
(c) linear sequence of amino acid residues in the polypeptide chain.
(d) regular folding patterns of continuous portions of the polypeptide chain.
Answer
D
Question. a-helix and b-pleated structures of proteins are classified as
(a) primary structure
(b) secondary structure
(c) tertiary structure
(d) quaternary structure
Answer
B
Question. Cheese is a
(a) globular protein
(b) conjugated protein
(c) denatured protein
(d) derived protein
Answer
C
5. When a solution of an a-amino acid is placed in an electric field depending on the pH of the medium, following three cases may happen.
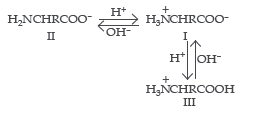
- In alkaline solution, a-amino acids exits as anion II, and there is a net migration of amino acid towards the anode.
- In acidic solution, a-amino acids exist as cation III, and there is a net migration of amino acids towards the cathode.
- If II and III are exactly balanced there is no net migration; under such conditions any one molecule exists as a positive ion and as a negative ion for exactly the same amount of time, and any small movement in the direction of one electrode is subsequently cancelled by an equal movement back toward the other electrode. The pH of the solution in which a particular amino acid does not migrate under the influence of an electric field is called the isoelectric point of that amino acid.
Answer the following MCQs by choosing the most appropriate options:
Question. In aqueous solutions, amino acids mostly exist as
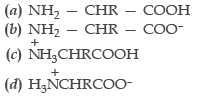
Answer
D
Question. The pKa1 and pKa2 of an amino acids are 2.3 and 9.7 respectively. The isoelectric points of the acids is
(a) 12.0
(b) 7.4
(c) 6.0
(d) 3.7
Answer
C
Question. Amino acids are least soluble
(a) at pH 1
(b) at pH 7
(c) at their isoelectric points
(d) none of these
Answer
C
Question.
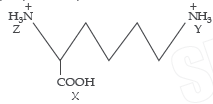
Arrange in order of increasing acid strengths.
(a) X > Z >Y
(b) Z < X < Y
(c) X > Y > Z
(d) Z > X > Y
Answer
A
Question. A tripeptide (X) on partial hydrolysis gave two dipeptides Cys-Gly and Glu-Cys. Identify the tripeptide.
(a) Glu-Cys-Gly
(b) Gly-Glu-Cys
(c) Cys-Gly-Glu
(d) Cys-Glu-Gly
Answer
A
6. Adenosine triphosphate (ATP) is the energycarrying molecule found in the cells of all living things. ATP captures chemical energy obtained from the breakdown of food molecules and releases it to fuel other cellular processes. ATP is a nucleotide that consists of three main structures: the nitrogenous base, adenine; the sugar, ribose; and a chain of three phosphate groups bound to ribose. The phosphate tail of ATP is the actual power source which the cell taps. Available energy is contained in the bonds between the phosphates and is released when they are broken, which occurs through the addition of a water molecule (a process called hydrolysis). Usually only the outer phosphate is removed from ATP to yield energy; when this occurs ATP is converted to adenosine diphosphate (ADP), the form of the nucleotide having only two phosphates. The importance of ATP (adenosine triphosphate) as the main source of chemical energy in living matter and its involvement in cellular processes has long been recognized. The primary mechanism whereby higher organisms, including humans, generate ATP is through mitochondrial oxidative phosphorylation. For the majority of organs, the main metabolic fuel is glucose, which in the presence of oxygen undergoes complete combustion to CO2 and H2O:
C6H12O6 + 6O2 → 6O2 + 6H2O + energy The free energy (ΔG) liberated in this exergonic (ΔG is negative) reaction is partially trapped as ATP in two consecutive processes: glycolysis (cytosol) and oxidative phosphorylation (mitochondria). The first produces 2 mol of ATP per mol of glucose, and the second 36 mol of ATP per mol of glucose. Thus, oxidative phosphorylation yields 17-18 times as much useful energy in the form of ATP as can be obtained from the same amount of glucose by glycolysis alone The efficiency of glucose metabolism is the ratio of amount of energy produced when 1 mol of glucose oxidised in cell to the enthalpy of combustion of glucose. The energy lost in the process is in the form of heat. This heat is responsible for keeping us warm.
Answer the following MCQs by choosing the most appropriate options:
Question. What is the efficiency of glucose metabolism if 1 mole of glucose gives 38 ATP energy?(Given:The enthalpy of combustion of glucose is 686 kcal, 1ATP= 7.3 kcal)
(a) 100%
(b) 38%
(c) 62%
(d) 80%
Answer
B
Question. Cellular oxidation of glucose is a:
(a) spontaneous and endothermic process
(b) non–spontaneous and exothermic process
(c) non–spontaneous and endothermic process
(d) spontaneous and exothermic process
Answer
D
Question. Which of the following statement is true?
(a) ATP is a nucleoside made up of nitrogenous base adenine and ribose sugar.
(b) ATP consists the nitrogenous base, adenine and the sugar, deoxyribose.
(c) ATP is a nucleotide which contains a chain of three phosphate groups bound to ribose sugar.
(d) The nitrogenous base of ATP is the actual power source.
Answer
C
Question. Which of the following statements is correct:
(a) ATP is a nucleotide which has three phosphate groups while ADP is a nucleoside which three phosphate groups.
(b) ADP contains a nitrogenous bases adenine, ribose sugar and two phosphate groups bound to ribose.
(c) ADP is the main source of chemical energy in living matter.
(d) ATP and ADP are nucleosides which differ in number of phosphate groups.
Answer
B
Question. Nearly 95% of the energy released during cellular respiration is due to:
(a) glycolysis occurring in cytosol.
(b) oxidative phosphorylation occurring in cytosol.
(c) glycolysis in occurring mitochondria.
(d) oxidative phosphorylation occurring in mitochondria.
Answer
D
7. Polysaccharides may be very large molecules. Starch, glycogen, cellulose, and chitin are examples of polysaccharides. Starch is the stored form of sugars in plants and is made up of amylose and amylopectin (both polymers of glucose). Amylose is soluble in water and can be hydrolyzed into glucose units breaking glycocidic bonds, by the enzymes α- amylase and β-amylase. It is straight chain polymer. Amylopectin is a branched chain polymer of several D-glucose molecules. 80% of amylopectin is present in starch. Plants are able to synthesize glucose, and the excess glucose is stored as starch in different plant parts, including roots and seeds. The starch that is consumed by animals is broken down into smaller molecules, such as glucose. The cells can then absorb the glucose.Glycogen is the storage form of glucose in humans and other vertebrates, and is made up of monomers of glucose. It is structurally quite similar to amylopectin. Glycogen is the animal equivalent of starch. It is stored in liver and skeletal muscles. Cellulose is one of the most abundant natural biopolymers. The cell walls of plants are mostly made of cellulose, which provides structural support to the cell. Wood and paper are mostly cellulosic in nature.
Like amylose, cellulose is a linear polymer of glucose. Cellulose is made up of glucose monomers that are linked by bonds between particular carbon atoms in the glucose molecule. Every other glucose monomer in cellulose is flipped over and packed tightly as extended long chains. This gives cellulose its rigidity and high tensile strength—which is so important to plant cells. Cellulose passing through our digestive system is called dietary fiber.
Answer the following MCQs by choosing the most appropriate options:
Question. Amylose is:
(a) straight chain, water insoluble component of starch, which constitutes 20 % of it .
(b) straight chain, water soluble component of starch, which constitutes 20 % of it.
(c) branched chain, water insoluble component of starch, which constitutes 80 % of it.
(d) branched chain, water soluble component of starch, which constitutes 80 % of it .
Answer
B
Question. Cellulose on complete hydrolysis yields:
(a) amylose
(b) amylopectin
(c) glucose
(d) amylose and amylopectin
Answer
C
Question. Which biopolymer breaks down to release glucose, whenever glucose levels drop in Our body:
(a) starch
(b) cellulose
(c) chitin
(d) glycogen
Answer
D
Question. In animals, Glycogen is stored in:
(a) Liver
(b) Spleen
(c) Lungs
(d) Small Intestine
Answer
A
Question. The linkages which join monosaccharides to form long chain polysaccharides:
(a) Peptide linkage
(b) Disulphide bonds
(c) Hydrogen bonds
(d) Glycosidic linkage
Answer
D
8. EVIDENCE FOR THE FIBROUS NATURE OF DNA. The basic chemical formula of DNA is now well established. As shown in Figure 1 it consists of a very long chain, the backbone of which is made up of alternate sugar and phosphate groups, joined together in regular 3′ 5′ phosphate di-ester linkages. To each sugar is attached a nitrogenous base, only four different kinds of which are commonly found in DNA. Two of these— adenine and guanine— are purines, and the other two thymine and cytosine—are pyrimidines. A fifth base, 5-methyl cytosine, occurs in smaller amounts in certain organisms, and a sixth, 5-hydroxy-methyl-cytosine, is found instead of cytosine in the T even phages. It should be noted that the chain is unbranched, a consequence of the regular internucleotide linkage. On the other hand the sequence of the different nucleotides is, as far as can be ascertained, completely irregular. Thus, DNA has some features which are regular, and some which are irregular. A similar conception of the DNA molecule as a long thin fiber is obtained from physicochemical analysis involving sedimentation, diffusion, light scattering, and viscosity measurements. These techniques indicate that DNA is a very asymmetrical structure approximately 20 A wide and many thousands of angstroms long. Estimates of its molecular weight currently center between 5 × 106 and 107 (approximately 3 × 104 nucleotides). Surprisingly each of these measurements tend to suggest that the DNA is relatively rigid, a puzzling finding in view of the large number of single bonds (5 per nucleotide) in the phosphate-sugar back bone. Recently these indirect inferences have been confirmed by electron microscopy.
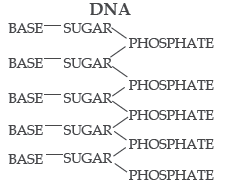
Chemical formula (diagrammatic) of a single chain of desoxyribonucleic acid.
Answer the following MCQs by choosing the most appropriate options:
Question. DNA molecule has …………….. internucleotide linkage and …………….. sequence of the different nucleotides.
(a) regular, regular
(b) regular, irregular
(c) irregular, regular
(d) irregular, irregular
Answer
B
Question. Purines present in DNA are:
(a) adenine and thymine
(b) guanine and thymine
(c) cytosine and thymine
(d) adenine and guanine
Answer
D
Question. Out of the four different kinds of nitrogenous bases which are commonly found in DNA, ………….. has been replaced in some organisms.
(a) adenine
(b) guanine
(c) cytosine
(d) thymine
Answer
C
Question. DNA has a backbone.
(a) phosphate -purine
(b) pyrimidines- sugar
(c) phosphate- sugar
(d) purine- pyrimidine
Answer
C
Very Short Answer Type Questions
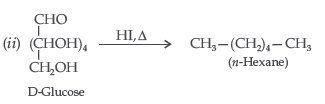
Question. Write a reaction which shows that all the carbon atoms in glucose are linked in a straight chain.
Answer. On prolonged heating with HI, it forms n-hexane, shows that all the six carbon atoms are linked in a straight chain :

Question. Name the only vitamin which can be synthesized in our body. Name the disease caused due to the deficiency of this vitamin.
Answer. Vitamin which can be synthesized in our body : Vitamin A Its deficiency causes Xerophthalmia.
Question. Where does the water present in the egg go after boiling the egg?
Answer. Denaturation of proteins is a process that changes the physical and biological properties of proteins without affecting the chemical composition of protein. In an egg, denaturation of protein is the coagulation of albumin present in the white of an egg. When an egg is boiled in water, the globular proteins present in it change to a rubber like insoluble mass which absorbs all the water present in the egg by making hydrogen bond with it.
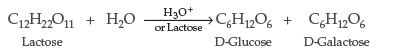
Question. Write the products of hydrolysis of lactose.
Answer. Lactose on hydrolysis with dilute acids gives an equimolar mixture of D-glucose and D-galactose.

Question. What are three types of RNA molecules which perform different functions?
Answer. m–RNA, t-RNA, r-RNA
Question. What is a glycosidic linkage?
Answer. The two monosaccharide units are joined together through an etheral or oxide linkage formed by loss of a molecule of water. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
Question. What are the expected products of hydrolysis of lactose ?
Answer. On hydrolysis, lactose gives β-D-galactose and β-D-glucose.
Question. What are enzymes?
Answer. Enzymes are protein molecules which act as catalyst in biochemical reaction.
Question. Define a ‘Peptide linkage’.
Answer. Peptide linkage : It is an amide linkage formed between – COOH group of one α-amino acid and NH2 group of the other α-amino acid by loss of a molecule of water. – CO – NH – bond is called Peptide linkage.
Question. Write the structure of the product obtained when glucose is oxidised with nitric acid.
Answer.

Question.Mention one important function of nucleic acids in our body.
Answer. Function of nucleic acid : Nucleic acids control the transmission of hereditary characters from one generation to another.
Question. Which component of starch is a branched polymer of α-glucose and insoluble in water?
Answer. Amylopectin.
Question. Name the products of hydrolysis of sucrose.
Answer. Glucose and fructose are the products of hydrolysis of sucrose.
Question. Name one water soluble vitamin which is a powerful antioxidant. Give its one natural source.
Answer. Water soluble vitamin : Vitamin C Natural source : Amla
Short Answer Type Questions-I
Question. How would you explain the amphoteric behavior of amino acids?
Answer.Amino acids are amphoteric due to the presence of both acidic and basic functional groups.
Question. Name the linkage present in proteins.
Answer. Peptide linkage
Question. Give the significance of prefix ‘D’ in the name D- (+)-glucose.
Answer.‘D’ Signifies that –OH group on C-5 is on the right-hand side
Question. How many asymmetric carbon atoms are present in D (+) glucose?
Answer. 4
Question. Write the Zwitter ion form of amino acetic acid. (H2NCH2COOH).
Answer.NH3+CH2COO–
Question. Mention the number of hydrogen bonds between adenine and thymine.
Answer.Two
Question. What type of linkage holds together the monomers of DNA and RNA?
Answer.Phosphodiester linkage.
Question. Give the significance of (+)-sign in the name D- (+)-glucose.
Answer. (+) sign indicates dextrorotatory nature of glucose.
Question.Which nucleic acid is responsible for carrying out protein synthesis in the cell?
Answer.RNA
Question.The two strands in DNA are not identical but complementary. Explain.
Answer. complementary bases are prepared.
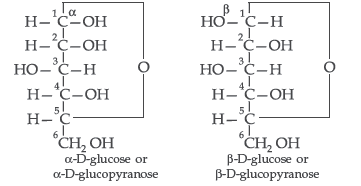
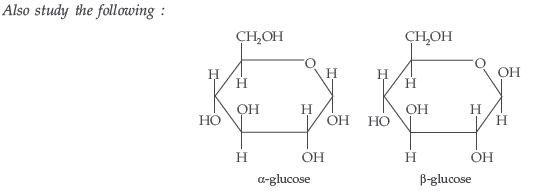
Question. What is essentially the difference between α-form of glucose and β-form of glucose? Explain.
Answer. In α-D-glucose, the OH group at C1 is towards right while in β-D-glucose, the OH group at C1 is towards left.
Question. Explain the following terms :
(i) Invert sugar (ii) Polypeptides
Answer. (i) Invert sugar : An equimolar mixture of glucose and fructose obtained by hydrolysis of sucrose in presence of an acid such as dil. HCl or the enzyme invertase or sucrase is called invert sugar.
(ii) Polypeptides : They are formed when several molecules of α-amino acids are joined together by peptide bonds.
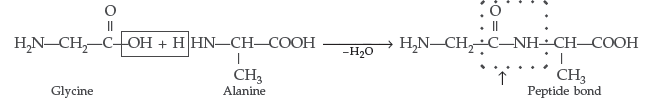
Question. Explain what is meant by
(i) a peptide linkage (ii) a glycosidic linkage.
Answer. (i) Peptide linkage : A peptide linkage is an amide linkage formed between – COOH group of one α-amino acid and NH2 group of the other α-amino acid by loss of a molecule of water.

(ii) Glycosidic linkage : The two monosaccharide units are joined together through an etheral or oxide linkage formed by loss of a molecule of water. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
Question. What are essential and non-essential amino acids in human food? Give one example of each type.
Answer. Essential amino acids : Amino acids which the body cannot synthesize are called essential amino acids.
Example : Valine, leucine etc. Therefore they must be supplied in diet.
Non-essential amino acids : Amino acids which the body can synthesize are called non-essential amino acids.Therefore, they may or may not be present in diet.
Example : Glycine, alanine etc.
Question. Write down the structures and names of the products formed when D-glucose is treated with
(i) Bromine water
(ii) Hydrogen Iodide (Prolonged heating).
Answer.

Question. Describe what you understand by primary structure and secondary structure of proteins.
Answer. Primary structure of proteins : Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence which is known as primary structure of protein.
Secondary structure of proteins : The conformation which the polypeptide chains assume as a result of hydrogen bonding is called the secondary structure of the protein.
Depending upon the size of the R groups, the two different secondary structures are possible which are :
(i) α-Helix structure : Intramolecular H–bonds present between the C = O of one amino acid and N – H of fourth amino acid.
(ii) β-Pleated sheet structure : The two neighbouring polypeptide chains are held together by intermolecular H–bonds.
Question.Write such reactions and facts about glucose which cannot be explained by its open chain structure.
Answer. Limitations of the open chain structure of glucose :
(i) Glucose does not form NaHSO3 addition product. Despite having aldehyde-ammonia group, it does not respond to 2,4-DNP test and does not respond to Schiff’s reagent test.
(ii) Glucose penta acetate does not react with NH2OH due to absence of aldehydic group.
Question.Name the four bases present in DNA. Which one of these is not present in RNA?
Answer. The four bases present in DNA are :
(i) Adenine (A) (ii) Guanine (G) (iii) Cytosine (C) (iv) Thymine (T)
In RNA, Thymine (T) is absent. It has Uracil (U) in place of Thymine.
Question. Explain what is meant by the following :
(i) peptide linkage (ii) pyranose structure of glucose
Answer. (i) Peptide linkage : A peptide linkage is an amide linkage formed between – COOH group of one α-amino acid and NH2 group of the other α-amino acid by loss of a molecule of water.
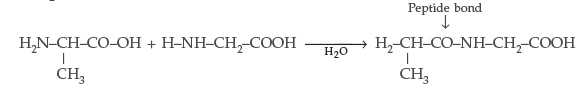
(ii) Pyranose structure of glucose : The six membered ring containing 5 carbon atoms and one oxygen atom because of its resemblance with pyron is called the pyranose form.
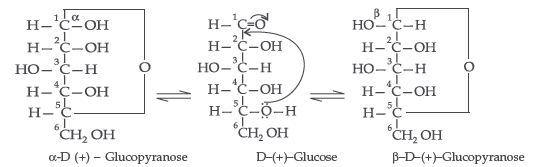
Question. Write the main structural difference between DNA and RNA. Of the four bases, name those which are common to both DNA and RNA.
Answer.
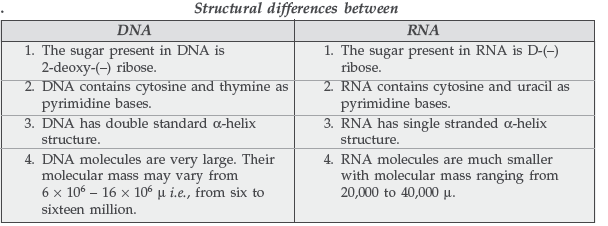
The bases which are common to both DNA and RNA are :
(i) Adenine (A) (ii) Guanine (G) (iii) Cytosine (C)
Question. State clearly what are known as nucleosides and nucleotides.
Answer. Nucleoside : A nucleoside contains only two basic components of nucleic acids i.e. a pentose sugar and a nitrogenous base. During their formation 1-position of the pyrimidine or 9-position of the purine moitey is linked to C1 of the sugar (ribose or deoxyribose) by a β-linkage.
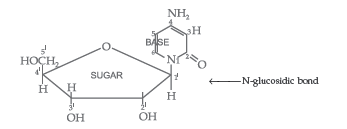
Nucleotides : A nucleotide contains all the three basic components of nucleic acids, i.e. a phosphoric acid group, a pentose sugar and a nitrogenous base. These are formed by esterification of C5′ – OH of the sugar of the nucleoside with phosphoric acid.
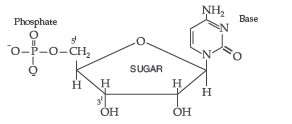
Question. Write the ambident nucleophiles? Give an example.
Answer. A group containing two nucleophilic centres.
Example : –CN (Cyanide) and –NC (Isocynide).
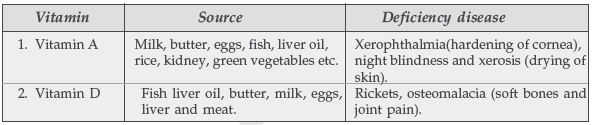
Question. Name two water soluble vitamins, their sources and the diseases caused due to their deficiency in diet.
Answer.

Question. Name the products of hydrolysis of sucrose. Why is sucrose not a reducing sugar?
Answer.

Sucrose is not a reducing sugar because it is unable to reduce Tollen’s reagent or Fehling solution.
Question. Write down the structures and names of the products formed when D-glucose is treated with
(i) Hydroxylamine (ii) Acetic anhydride.
Answer.

Question. Name two fat soluble vitamins, their sources and the diseases caused due to their deficiency in diet.
Answer.
Question. (a) Name the only vitamin which can be synthesized in our body. Name one disease that is caused due to the deficiency of this vitamin.
(b) State two functions of carbohydrates.
Answer. (a) Vitamin that can be synthesized :Vitamin B12 Disease due to the deficiency of Vitamin B12 : Pernicious anaemia.
(b) Two functions of glucose :
(i) Carbohydrates such as glucose, starch, glycogen etc. provide energy for functioning of living organisms.
(ii) Carbohydrates, especially cellulose in the form of wood is used for making furniture, houses etc. by us.
Question. Name the bases present in RNA. Which one of these is not present in DNA?
Answer. The four bases present in RNA are :
Purines – Adenine (A) and Guanine (G)
Pyrimidines – Uracil (U) and Cytosine (C)
Uracil is not present in DNA.
Question. Answer the following questions:
(i) Why are vitamin A and vitamin C essential for us?
(ii) What is the difference between a nucleoside and a nucleotide?
Answer. (i) Because deficiency of vitamin A and vitamin C causes night blindness and scurvy respectively.
Question. Write the main structural difference between DNA and RNA. Of the two bases, thymine and uracil, which one is present in DNA?
Answer. (i) Difference between DNA and RNA :
(ii) Thymine is present in DNA.
Question. Enumerate the reactions of glucose which cannot be explained by its open chain structures.
Answer. Limitations of the open chain structure of glucose :
(i) Glucose does not form NaHSO3 addition product. Despite having aldehyde-ammonia group, it does not give 2,4-DNP test and does not respond to Schiff’s reagent test.
(ii) Glucose penta acetate does not react with NH2OH due to absence of aldehydic group.
Question. Write any two reactions of glucose which cannot be explained by the open chain structure of glucose molecule.
Answer. (i) Despite having the aldehyde group, glucose does not give 2, 4-DNP test or Schiff’s test.
(ii) It does not form the hydrogen sulphite addition product with NaHSO3.
(iii) The pentaacetate of glucose does not react with hydroxylamine indicating the absence of free – CHO group.
Short Answer Type Questions-II
Question. Why are carbohydrates generally optically active?
Answer. It is due to the presence of Chiral Carbon atoms in their molecules
Question. Explain tertiary structure of Protein.
Answer. Tertiary structure of proteins: The tertiary structure of proteins represents overall folding of the polypeptide chains i.e., further folding of the secondary structure. It gives rise to two major molecular shapes viz. fibrous and globular. The main forces which stabilize the 2° and 3° structures of proteins are hydrogen bonds, disulphide linkages, van der Waals and electrostatic force of attraction.

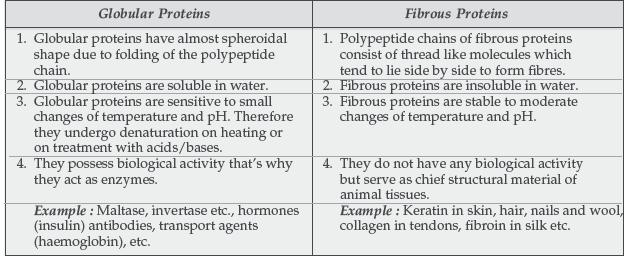
Question. How is globular protein different from fibrous protein?
Answer.

Question. What is difference between reducing and non‐reducing sugars or carbohydrates?
Answer. All those carbohydrates which contain aldehydic and ketonic group in the hemiacetal or hemiketal form and reduce Tollen’s reagent or Fehling’s solution are called reducing carbohydrates while others which do not reduce these reagents are called non‐reducing sugars.
Question. Differentiate between fibrous proteins and globular proteins. What is meant by the denaturation of a protein?
Answer.
Denaturation of protein : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.
Question. (a) Write the structural and functional differences between DNA and RNA
(b) Name two components of starch.
Answer. Functional difference : DNA’s main function is to control cell activities like telling each organ what to make and what to do. RNA’s main function is to make protein.
(b) Components of starch : Amylose and amylopectin.
Question. What is essentially the difference between α-glucose and β-glucose? What is meant by pyranose structure of glucose?
Answer. The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group on the first carbon atom called anomeric carbon. Such isomers i.e. α-form and β-form are called anomers. α-glucose is the monomer unit of starch and β-glucose is the monomer unit of cellulose. The six membered cyclic structure of glucose is called pyranose structure.
Question. (i) Write the structural difference between starch and cellulose.
(ii) What type of linkage is present in Nucleic acids?
(iii) Give one example each for fibrous protein and globular protein.
Answer. (i) Starch contains the α-D-glucose as its monomer units while cellulose contains β-Dglucose as its monomer units.
(ii) Phosphodiester linkages are present in Nucleic Acids
(iii) Globular protein : All enzymes and hormones like insulin.
Fibrous protein : Keratin in skin.
Question. Define the following as related to proteins :
(i) Peptide linkage (ii) Primary structure (iii) Denaturation
Answer. (i) Peptide linkage : A peptide linkage is an amide linkage formed between – COOH group of one α-amino acid and NH2 group of the other α-amino acid by loss of a molecule of water. The–CO–NH–bond formed is called petide linkage.
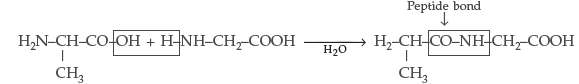
(ii) Primary structure : Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is called the primary structure of that protein.
(iii) Denaturation : Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the formed protein is called denaturated proteins and the phenomenon is denaturation.
Question. (i) Deficiency of which vitamin causes rickets?
(ii) Give an example for each of fibrous protein and globular protein.
(iii) Write the product formed on reaction of D-glucose with Br2 water.
Answer. (i) Deficiency of Vitamin D causes rickets.
(ii) Fibrous protein → α-keratin
Globular protein → Insulin
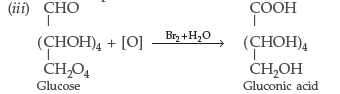
Question. (a) Give two differences between globular and fibrous proteins.
(b) What change occurs in the nature of egg protein on boiling?
Answer.
(b) Because the egg comes in contact with a solution of higher osmotic pressure, the egg will shrink due to going out of water. This shrinking of egg is called plasmolysis.
Question. Define the following terms :
(i) Nucleotide (ii) Anomers (iii) Essential amino acids
Answer. (i) Nucleotide : A nucleotide contains all the three basic components of nucleic acids, i.e. a phosphoric acid group, a pentose sugar and a nitrogenous base. These are formed by esterification of C5′ – OH of the sugar of the nucleoside with phosphoric acid.
Structure of Nucleotide:
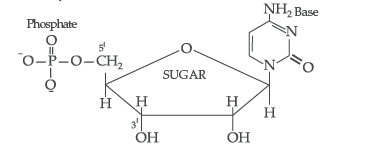
(ii) Anomers : A pair of stereoisomers which differ in configuration only around C1 are called anomers. Two isomers are said to be anomers if the isomerisation in the molecule is at first carbon.
Question. What are essential and non-essential amino acids? Give two examples of each.
Answer. Essential amino acids : Amino acids which the body cannot synthesize are called essential amino acids.
Example : Valine, leucine etc. Therefore they must be supplied in diet. Non-essential amino acids : Amino acids which the body can synthesize are called non-essential amino acids. Therefore, they may or may not be present in diet.
Example : Glycine, alanine etc.
Question. (i) Which one of the following is a polysaccharide :
Starch, Maltose, Fructose, Glucose?
(ii) What one difference between α-helix and β-pleated sheet structure of protein.
(iii) Write the name of the disease caused by the deficiency of Vitamin B12.
Answer. (i) Starch is a polysaccharide.
(ii) α-Helix structure : The polypeptide chains are held together (stabilized) by intramolecular H-bonding. β-Pleated sheet structure : The two neighbouring polypeptide chains are held together by intermolecular H–bonding.
(iii) Disease caused by the deficiency of Vitamin B12 is Pernicious anaemia.
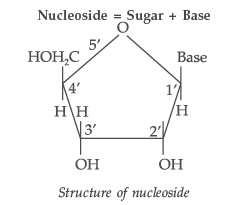
Nucleotides. A nucleotide contains all the three basic components of nucleic acids, i.e., a phosphoric acid group, a pentose sugar and nitrogenous base. These are formed by the esterification of C5—OH of the sugar of the nucleoside with phosphoric acid.
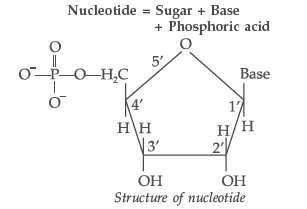
Question. Define the following terms :
(i) Glycosidic linkage (ii) Invert sugar (iii) Oligosaccharides
Answer. (i) Glycosidic linkage : The two monosaccharide units are joined together through an etheral or oxide linkage formed by loss of a molecule of water. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage
(ii) Invert sugar : An equimolar mixture of glucose and fructose obtained by hydrolysis of sucrose in presence of an acid such as dil. HCl or the enzyme invertase or sucrase is called invert sugar.
(iii) Oligosaccharides : Those carbohydrates which on hydrolysis give 2-10 molecules of monosaccharides are called oligosaccharides.
Example : sucrose, maltose.