Students should refer to Worksheets Class 12 Chemistry Alcohols Phenols and Ethers Chapter 11 provided below with important questions and answers. These important questions with solutions for Chapter 11 Alcohols Phenols and Ethers have been prepared by expert teachers for Class 12 Chemistry based on the expected pattern of questions in the Class 12 exams. We have provided Worksheets for Class 12 Chemistry for all chapters on our website. You should carefully learn all the important examinations questions provided below as they will help you to get better marks in your class tests and exams.
Alcohols Phenols and Ethers Worksheets Class 12 Chemistry
Multiple Choice Questions (MCQs)
Question. Which compound is predominantly formed when phenol is allowed to react with bromine in aqueous medium?
(a) Picric acid
(b) O-Bromophenol
(c) 2, 4, 6-Tribromophenol
(d) p-Bromophenol
Answer
C
Question. The correct acidic strength order of the following:

is—
(a) I > II > III
(b) III > I > II
(c) II > III > I
(d) I > III > II
Answer
B
Question. The correct order of boiling point of primary (1°), secondary (2°) and tertiary (3°) alcohols is
(a) 1° > 2° > 3°
(b) 3° > 2° > 1°
(c) 2° > 1° > 3°
(d) 2° > 3° > 1°
Answer
A
Question. Phenols are more acidic than alcohols because
(a) Phenoxide ion is stablised by resonance
(b) Phenols are more soluble in polar solvents
(c) Phenoxide ion does not exhibit resonance
(d) Alcohols do not lose H atoms at all
Answer
A
Question. C2H5OH + SOCl2 Pyridine→ C2H5Cl + SO2 + HCl
(a) Williamson’s reaction.
(b) Hofmann’s synthesis.
(c) Mendis reaction.
(d) Darzen’s reaction.
Answer
D
Question. The compound B is formed in the sequence of the reaction given below:
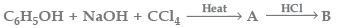
The compound B is
(a) Salicylaldehyde
(b) Benzoic acid
(c) Salicylic acid
(d) Cinnamic acid
Answer
C
Question. The major organic product in the reaction,
CH3 — O — CH(CH3)2 + HI → product: is/are

Answer
A
Question. Identify Z in the series
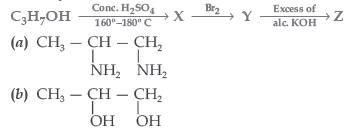
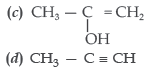
Answer
D
Question. 1-propanol and 2-propanol can be best distinguished by
(a) Oxidation with KMnO4 followed by reaction with Fehling solution.
(b) Oxidation with acidic dichromate followed by reaction with Fehling solution.
(c) Oxidation by heating with copper followed by reaction with Fehling solution.
(d) Oxidation with concentrated H2SO4 followed by reaction with Fehling solution.
Answer
C
Question. Which of the following reagents cannot be used to distinguish between phenol and benzyl alcohol?
(a) FeCl3
(b) Litmus soln
(c) Br2/CCl4
(d) All of the above
Answer
C
Assertion-Reason Questions
DIRECTION: Mark the option which is most suitable:
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
Question. Assertion: Phenol is more acidic than ethanol.
Reason: Phenoxide ion is resonance stabilised.
Answer
A
Question. Assertion: tert-Butyl alcohol undergoes acid catalysed dehydration readily than propanol.
Reason: 3º Alcohols do not give Victor-Meyer’s test.
Answer
B
Question. Assertion: Boiling points of alcohols are lower than hydrocarbons.
Reason: Among isomeric alcohols, boiling point decreases in the order: 1º > 2º > 3º.
Answer
D
Question. Assertion: o-Nitrophenol is more volatile than p-nitrophenol.
Reason: Intramolecular hydrogen bonding is present in o-nitrophenol while intermolecular H-bonding is in p-nitrophenol.
Answer
A
Question. Assertion: Phenol decomposes NaHCHO3 solution to evolve CO2 gas.
Reason: Picric acid is 2, 4, 6-trinitrophenol.
Answer
D
Question. Assertion: Phenol is less acidic than p-nitrophenol.
Reason: Phenolate ion is more stable than p-nitrophenol ion.
Answer
C
Question. Assertion: Primary and secondary alcohols can be distinguished by Victor-Meyer’s test.
Reason: Primary alcohols form nitrolic acid which dissolves NaOH to form blood red colouration but secondary alcohols form pseudonitrots which give blue colouration with NaOH.
Answer
A
Question. Assertion: With Br2—H2O, phenol gives 2,4,6-tribromophenol but with Br2—CS2, it gives 4-bromophenol as the major product.
Reason: In water, ionisation of phenol is enhanced but in CS2, it is greatly suppressed.
Answer
A
Question. Assertion: Solubility of alcohols decreases with increase in size of alkyl/aryl groups.
Reason: Alcohols form H-bonding with water to show soluble nature.
Answer
B
Question. Assertion: Reimer–Tiemann reaction of phenol with CHCl3 in NaOH at 340 K gives salicylic acid as the major product.
Reason: The reaction occurs through intermediate formation of +CHCl2.
Answer
C
Case Based Questions
1. A compound (X) containing C, H and O is unreactive towards sodium. It also does not react with Schiff’s reagent. On refluxing with an excess of hydroiodic acid, (X) yields only one organic product (Y). On hydrolysis, (Y) yields a new compound (Z) which can be converted into (Y) by reaction with red phosphorus and iodine. The compound (Z) on oxidation with potassium permanganate gives a carboxylic acid. The equivalent weight of this acid is 60.
Answer the following questions by choosing the most appropriate options:
Question. The IUPAC name of the acid formed is
(a) methanoic acid
(b) ethanoic acid
(c) propanoic acid
(d) butanoic acid
Answer
B
Question. Compound (X) is
(a) ethyl iodide
(b) methyl iodide
(c) propyl iodide
(d) mixture of (a) and (b)
Answer
A
Question. Compound (X) on treatment with excess of Cl2 in the presence of light gives
(a) a-chlorodiethyl
(b) a’-dichlorodiethyl ether
(c) perchlorodiethyl ether
(d) none of above
Answer
C
Question. The compound (X) is an
(a) acid
(b) aldehyde
(c) alcohol
(d) ether
Answer
D
Question. Compound (Z) is
(a) methanol
(b) ethanol
(c) propanol
(d) butanol
Answer
B
2. Both alcohols and phenols are acidic in nature, but phenols are more acidic than alcohols. Acidic strength of alcohols mainly depends upon the inductive effect. Acidic strength of phenols depends upon a combination of both inductive effect and resonance effects of the substituent and its position on the benzene ring. Electron withdrawing groups increase the acidic strength of phenols whereas electron donating groups decrease the acidic strength of phenols. Phenol is weaker acid than carboxylic acid.
Answer the following questions by choosing the most appropriate options:
Question. The compound that does not liberate CO2, on treatment with aqueous sodium bicarbonate solution is
(a) benzoic acid
(b) benzenesulphonic acid
(c) salicylic acid
(d) carbolic acid
Answer
C
Question. The correct order of acidic strength among the following is:
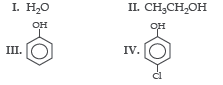
(a) (III) > (IV) > (II) > (I)
(b) (IV) > (III) > (I) > (II)
(c) (IV) > (III) > (II) > (I)
(d) (I) > (II) > (IV) > (III)
Answer
B
Question. The correct decreasing order of pKa value is
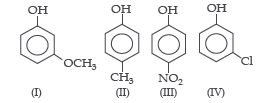
(a) II > IV > I > III
(b) IV > II > III > I
(c) III > II > IV > I
(d) IV > I > II > III
Answer
A
Question. Phenols are highly acidic as compared to alcohols due to
(a) the higher molecular mass of phenols
(b) the stronger hydrogen bonds in phenols
(c) alkoxide ion is a strong conjugate base
(d) phenoxide ion is resonance stabilised.
Answer
D
Question. Most acidic amongst the following is
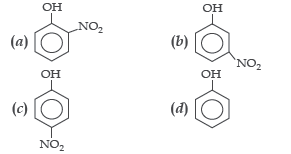
Answer
C
3. An organic compound (A) having molecular formula C6H6O gives a characteristic colour with aqueous FeCl3 solution. (A) on treatment with CO2 and NaOH at 400 K under pressure gives (B), which on acidification gives a compound (C).The compound (C) reacts with acetyl chloride to give (D) which is a popular pain killer.
Answer the following questions by choosing the most appropriate options:
Question. Compound (A) on heating with compound (C) in presence of POCl3 gives a compound (D) which is used
(a) in perfumery as a flavouring agent
(b) as an antipyretic
(c) as an analgesic
(d) as an intestinal antiseptic
Answer
D
Question. Compound (C) is
(a) salicylic acid
(b) salicyladehyde
(c) benzoic acid
(d) benzaldehyde
Answer
A
Question. Number of carbon atoms in compound (D) is
(a) 7
(b) 6
(c) 8
(d) 9
Answer
D
Question. Compound (A) is
(a) 2-hexanol
(b) dimethyl ether
(c) phenol
(d) 2-methyl pentanol
Answer
C
Question.The conversion of compound (A) to (C) is known as
(a) Reimer-Tiemann reaction
(b) Kolbe’s reaction
(c) Smarts reaction
(d) Girgnard reaction
Answer
B
4. Reimer-Tiemann reaction introduces an aldehyde group, on aromatic ring of phenol, ortho to the hydroxyl group. This is a general method for the synthesis of substituted salicylaldehydes as depicted bleow.
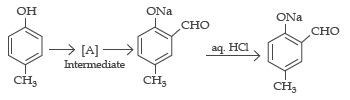
Answer the following questions by choosing the most appropriate options:
Question. The structure of the intermediate [A] is

Answer
B
Question. Reimer-Tiemann reaction is an example of
(a) nucleophilic substitution reaction
(b) electrophilic substitution reaction
(c) nucleophilic addition reaction
(d) electrophilic addition reaction
Answer
B
Question. When phenol reacts with chloroform in presence of KOH, the product formed is
(a) salicylic acid
(b) salicyladehyde
(c) both (a) and (b)
(d) none of these
Answer
B
Question. Which of the following reagents is used in the given reaction in steps I?
(a) aq. NaOH + CH3Cl
(b) aq. NaOH + CH2Cl2
(c) aq. NaOH + CHCl3
(d) aq. NaOH + CCl4
Answer
C
Question. The electrophilie in this reaction [A] is
(a) :CHCl
(b) +CHCl2
(c) :CCl2
(d) CCl3
Answer
C
5. Dehydration of alcohols can lead to the formation of either alkenes or ethers. This dehydration can be carried out either with protonic acids such as conc. H2SO4, H3PO4 or catalysts such as anhydrous ZnCl2 or Al2O3. When primary alcohols are heated with conc. H2SO4 at 433-443 K, they undergo intramolecular dehydration to form alkenes. Secondary and tertiary alcohols undergo dehydration under milder conditions. The ease of dehydration of alcohols follows the order : 3º > 2º > 1º.
The dehydration of alcohols always occurs in accordance with the Saytzeff’s rule. Primary alcohols when heated with protic acid at 413 K, give dialkyl either.
Answer the following questions by choosing the most appropriate options:
Question. Which one of the following alcohols undergoes acid-cataysed dehydration to alkenes most readily?
(a) (CH3)2CHCH2OH
(b) (CH3)3COH
(c) CH3CHOHCH3
(d) CH3CH2CH2OH
Answer
B
Question. The alcohol which does not give a stable compound on dehydration is
(a) ethyl alcohol
(b) methyl alcohol
(c) n-propyl alcohol
(d) n-butyl alcohol
Answer
B
Question.
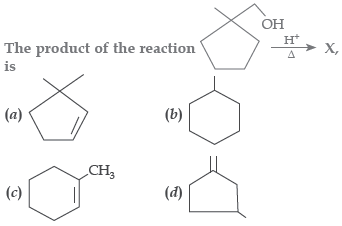
Answer
C
Question.
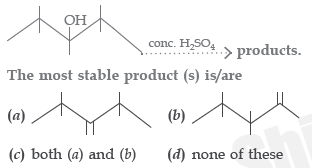
Answer
A
6. Alcohols are derivatives of hydrocarbons in which an –OH group has replaced a hydrogen atom. Although all alcohols have one or more hydroxyl (–OH) functional groups, they do not behave like bases such as NaOH and KOH. NaOH and KOH are ionic compounds that contain OH– ions. Alcohols are covalent molecules; the –OH group in an alcohol molecule is attached to a carbon atom by a covalent bon(d) The term alcohol originally referred to the primary alcohol ethanol(ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic beverages. Alcohols are compounds find wide application in industries( preparation of dyes, drugs, solvents etc) and are of immense use in our daily life. They contain one or more –OH group (responsible for the variation in their properties). They are classified as primary, secondary and tertiary alcohol. During chemical reaction they undergoes Substitution, Dehydration , Dehydrogenation. Dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At its simplest, it is useful way of converting alkanes, which are relatively inert and thus low-valued, to olefins, which are reactive and thus more valuable. Dehydrogenation of alcohols gives aldehyde, ketone and alkene , when alcohol is heated at 573K in the presence of copper.
Question. The product of which alcohol gives positive Tollen’s test –
(a) Primary alcohol
(b) Secondary alcohol
(c) Tertiary alcohol
(d) All of the abov
Answer
A
Question. Which among primary, secondary and tertiary alcohol on oxidation gives carboxylic acid having same number of carbon atom?
(a) Primary alcohol
(b) Secondary alcohol
(c) Tertiary alcohol
(d) Isopropyl alcohol
Answer
A
Question. An organic compound ‘A’ having molecular formula C6H4O is an isomer of alcohol with excess of HI forms two alkyl halides. On hydrolysis gives two compounds B and C . Oxidation B gives an acid D and C gives a mixed ketone. The name of D and E is-
(a) Propanoic acid, Butan-2one
(b) Ethanoic acid , Butan-2-one
(c) Butanoic acid , Propan-2-one
(d) Methanoic acid, 3Methyl But-2one
Answer
B
Question. In all the following isomeric alcohols with molecular formula C5H12O , identify the isomers exhibit chirality.
(a) Pentan-3-ol
(b) 2- Methyl butan-2-ol
(c) 3Methyl butan-2-ol
(d) 2,2 Dimethylpropan-1-ol.
Answer
C
7. “Lucas’ reagent” is a solution of anhydrous zinc chloride in concentrated hydrochloric aci(d) This solution is used to classify alcohols of low molecular weight. The reaction is a substitution in which the chloride replaces a hydroxyl group. A positive test is indicated by a change from clear and colorless to turbid, signaling formation of a chloroalkane. Also, the best results for this test are observed in tertiary alcohols, as they form the respective
alkyl halides fastest due to higher stability of the intermediate tertiary carbocation. The Lucas test in alcohols is a test to differentiate between primary, secondary, and tertiary alcohols. It is based on the difference in reactivity of the three classes of alcohols with hydrogen halides via an SN1 reaction. Iodoform (also known as triiodo methane and, inaccurately, as carbon triiodide) is the organ iodine compound with the formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant.
Question. Which of the above alcohol does not give positive iodoform test?
(a) Butan-1-ol
(b) Butan-2-ol
(c) 2-Methyl propal-1-ol
(d) 2- Methyl propan-2-ol
Answer
A
Question. Which carbonyl compound is used to prepare 2-Methyl propan2-ol with methyl magnesium bromide?
(a) Methanol
(b) Ethanal
(c) 2-Propanone
(d) 2- Butanone
Answer
C
Question. An unknown alcohol gives positive Lucas test in about 5 min. When this alcohol was heated with con(c) sulphuric acid alkene was formed with the formula C4H8. Ozonolysis of this alkene gives a single product C2H4O.Identify the alcohol-
(a) Butan-2-ol
(b) 2-Methyl propan-1-ol
(c) 2- Methyl propan-2-ol
(d) Butan-1-ol
Answer
A
Question. Which isomer of the unknown alcohol forms alkene on dehydrogenation?
(a) Butan-1-ol
(b) Butan-2-ol
(c) 2-Methyl propal-1-ol
(d) 2- Methyl propan-2-ol
Answer
D
Read the passage given below, and answer the following questions:
Alcohol is very important organic compound widely used in industry to prepare paints, varnishes, Medicines and different types Liquor.
Ethanol is the main constituents of different types of wines. Commercial alcohols is made unfit for drinking by mixing in it copper sulphate( to give it color ) and pyridine(a Foul smelling liquid) it is known as Denaturation of alcohols. Ingestion of small quantity of Methanol can cause blindness and large quantities cause even death.
Grignard Reagent can be used to prepare different types of alcohols. Methanol when treated with Grignard Reagent followed by hydrolysis forms primary alcohols. Ethanol and other aldehydes can be used to prepare Secondary alcohols. Ketones when treated with Grignard Reagent Followed by hydrolysis leads to the formation of tertiary alcohols.
In the following questions, a statement of assertion followed by a statement of reason is given
Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements. And reason is the correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not the correct explanation for assertion.
(c) Assertion is correct but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
Question. Assertion: Ethanol contaminated with methanol is not feet for drinking
Reason: Methanol is highly piousness for human body .
Answer
A
Question.Assertion : Grignard reagent can be used to prepare tertiary alcohol.
Reason: Grignard reagent do not react with Ketone
Answer
C
Question. Assertion: denaturation of commercial Alcohols can be done by mining copper sulphate and Pyridine.
Reason : Denatured alcohol is unfit for drinking.
Answer
B
Question. Assertion: Isopropyl Alcohol can be prepared by using grignard reagent
Reason : Methyl magnesium bromide reacts with ethanal followed by hydrolysis form isopropyl alcohol.
Answer
A
Nomenclature of Organic Compound
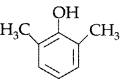
Question. Draw the structure of 2, 6-Dimethylphenol.
Answer.
Question. Write IUPAC name of the following
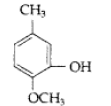
Answer. IUPAC name : 2-Methoxy-5-methyl phenol
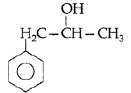
Question. Write the structure of the molecule of a compound whose IUPAC name is 1-phenylpropan-2-ol.
Answer. 1-phenylpropan-2-ol
Question. Write the IUPAC name of given compound:
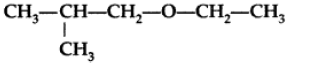
Answer. IUPAC name : 1-Ethoxy-2-methylpropane
Question. Give the IUPAC name of the following compound :
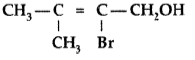
Answer. IUPAC name : 2-Bromo-3-methyl-but-2-ene-1-ol
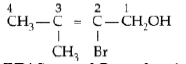
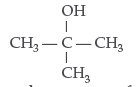
Question. Draw the structural formula of 2-methylpropan- 2-ol molecule.
Answer.
Question. Write the IUPAC name of the given compound
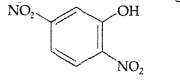
Answer. 2, 5-dinitrophenol.
Question. Write the IUPAC name of the following :
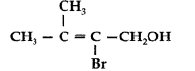
Answer. IUPAC name : 2-Bromo-3-methyl but-2-en-l-ol.
Question. Give the IUPAC name of the following
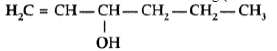
Answer. IUPAC name: Hex-1-en-2-ol or 3-Hexenol
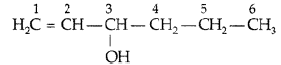
Question. Draw the structure of hex-l-en-3-ol compound.
Answer. CH2 = CH – CH(OH) – CH2 – CH2 – CH3
Very Short Answer Type Questions
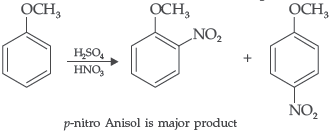
Question. Write equation of the nitration of anisole.
Answer.
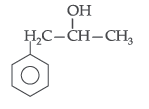
Question. Write the structure of the molecule of a compound whose IUPAC name is 1-phenylpropan-2-ol.
Answer. 1-phenylpropan-2-ol
Question. Give a chemical test to distinguish between 2-Pentanol and 3-Pentanol.
Answer. 2-pentanol gives Iodoform test with yellow ppt. of Iodoform while 3-pentanol does not give this test.
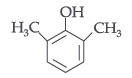
Question. Draw the structure of 2, 6-Dimethylphenol.
Answer.
Question. Draw the structure of hex-1-en-3-o1 compound.
Answer. CH2 = CH – CH(OH) – CH2 – CH2 – CH3
Question. Ortho nitrophenol has lower boiling point than p-nitrophenol. Why ?
Answer. Ortho-nitrophenol has lower boiling point due to formation of intramolecular H-bonding whereas p-nitrophenol forms intermolecular H-bonding.
Question. Name the alcohol that is used to make the following ester :
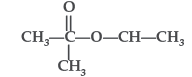
Answer. Alcohol used : Propan-2-ol
Question. The C–O bond is much shorter in phenol than in ethanol. Give reason.
Answer. Carbon of C–O bond of phenol is Sp2 hybridised, so it acquires a partial double bond character but in ethanol it is Sp3 hybridised and a single bond.Double bond is shorter than a single bond.
Question. Write the IUPAC name of the following :
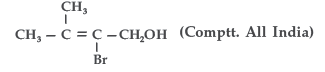
Answer. IUPAC name : 2-Bromo-3-methyl but-2-en-1-ol.
Question. Give simple chemical tests to distinguish between the following pairs of compounds: Benzoic acid and Phenol
Answer. Ferric chloride test. Add neutral FeCl3 in both the solutions, phenol reacts with neutral FeCl3 to form an iron-phenol complex giving violet colour but benzoic acid does not.
Question. Of the two hydroxy organic compounds ROH and R′OH, the first one is basic and other is acidic in behaviour. How is R different from R′?
Answer. When R = alkyl, ROH behaves as a bronsted base and when R′ = aryl, R′OH behaves as a bronsted acid.
Question. Out of CH3OH and
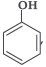
which one is more acidic?
Answer.

is more acidic, as Phenoxide formed is more stabilized by Resonance.
Question. What happens when phenol is oxidized by Na2Cr2O7/H2SO4?
Answer. Phenol forms benzoquinone on oxidation with Na2Cr2O7/H2SO4.

Question. How would you convert ethanol to ethene?
Answer.
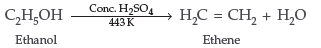
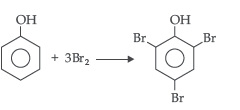
Question. What happens when phenol is treated with bromine water?
Answer. 2, 4, 6-tribromophenol is formed when phenol is treated with bromine water.
Question. Give the IUPAC name of the following compound :
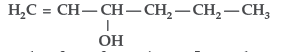
Answer.
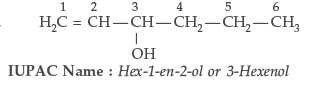
Question. Write IUPAC name of the following :
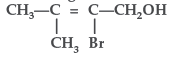
Answer. IUPAC name : 2–Bromo–3–methylbut –2–ene–1-ol
Question. How would you obtain phenol from benzene?
Answer.

Question. Write IUPAC name of the following compound :
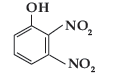
Answer. 2, 3-Dinitro phenol.
Question. Write IUPAC name of the following :
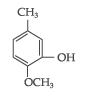
Answer. IUPAC name : 2–Methoxy–5–methyl phenol
Question. Give the IUPAC name of the following compound :
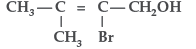
Answer.
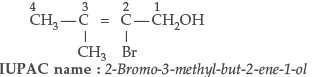
Question. Write the IUPAC name of the given compound.

Answer. 2, 5-dinitrophenol
Question. How would you obtain acetophenone from phenol?
Answer.

Question. Write the IUPAC name of the given compound:
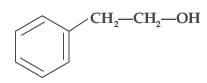
Answer. 2-Phenylethanol
Question.. How would you obtain ethane-1, 2-diol from ethanol?
Answer.
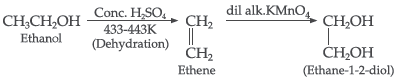
Question. Write the IUPAC name of the following compound:
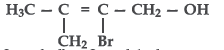
Answer. 2-Bromo-3-methylbut-2-enol-1-ol
Question. Which of the following isomers is more volatile : o-nitrophenol or p-nitrophenol? (Delhi)
Answer. o-nitrophenol is more volatile than p-nitrophenol due to intramolecular hydrogen bonding.
Question. Ortho-nitrophenol is more acidic than orthomethoxyphenol. Why?
Answer. NO2 group is an electron withdrawing group while methoxy group is electron donating in nature. The release of H+ is easier from O-nitrophenol while it is difficult from O-methoxyphenol.
Question. Write the IUPAC name of the following compound:
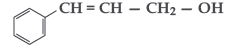
Answer. 3-phenylprop-2-en-1-ol
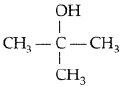
Question. Draw the structural formula of 2-methylpropan- 2-ol molecule.
Answer.
Question. Write the IUPAC name of the given compound:
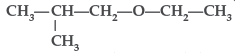
Answer. IUPAC name : 1-Ethoxy-2-methylpropane
Short Answer Type Questions-I
Question. How are the following conversions carried out?
(i) Propene to Propan-2-ol (ii) Ethyl chloride to Ethanal
Answer.

Question. Explain the following reactions with an example for each :
(i) Reimer-Tiemann reaction (ii) Friedel-Crafts reaction.
Answer. (i) Reimer–Tiemann reaction : Treatment of phenol with CHCl3 in presence of aqueous NaOH at 340K followed by hydrolysis gives salicylaldehyde.
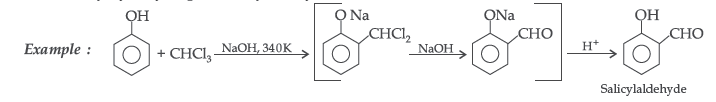
(ii) Friedel-Crafts reaction : This reaction is used for introducing an alkyl or an acyl group into an aromatic compound in presence of Lewis acid catalyst (AlCl3)
Example :

Question. Explain the mechanism of the following reaction:

Answer.

Question. How are the following conversions carried out?
(i) Benzyl chloride to benzyl alcohol,
(ii) Methyl magnesium bromide to 2-methylpropan-2-ol.
Answer.

Question. Explain the following giving one example for each :
(i) Reimer-Tiemann reaction. (ii) Friedel-Craft’s acetylation of anisole.
Answer.
(ii) Friedel-Craft’s acetylation of anisole :

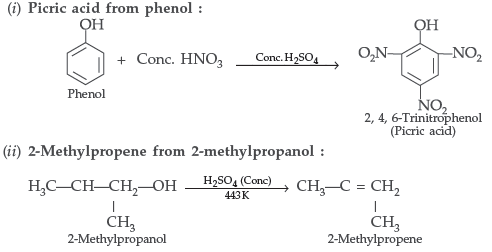
Question. How would you obtain
(i) Picric acid (2, 4, 6-trinitrophenol) from phenol,
(ii) 2-Methylpropene from 2-methylpropanol?
Answer.
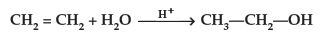
Question. Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol.
Answer. Acid catalysed hydration : Alkenes react with water in the presence of acid as catalyst to form alcohols
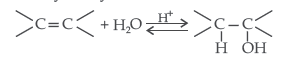
Mechanism : It involves three steps :
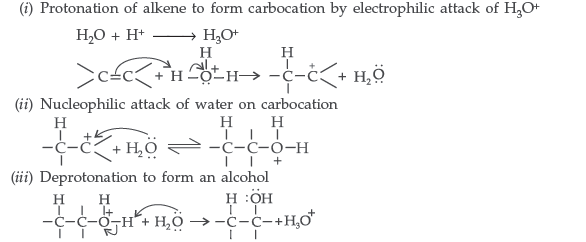
Question. (a) Arrange the following compounds in the increasing order of their acid strength: p-cresol, p-nitrophenol, phenol
(b) Write the mechanism (using curved arrow notation) of the following reaction:

Answer. (a) Order of acidic strength: p-cresol < phenol < p-nitrophenol
(b) Mechanism of acid catalysed hydration of alkene:
Step 1: Protonation of alkene to form carbocation by electrophilic attack of H3O+.

Question. Explain the following behaviours :
(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.
(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Answer. (i) Alcohols can form H-bonds with water and break the H-bonds already existing between water molecules. So they are soluble in water.
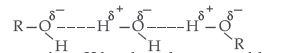
On the other hand, hydrocarbons cannot form H-bonds with water and hence are insoluble in water.
(ii) Due to strong – R and – I effect of the – NO2 group, electron density in the – OH bond decreases and hence the loss of a proton becomes easier. Moreover O-nitrophenoxide ion is stabilized by resonance,
thereby making O-nitrophenol a stronger acid.
In O-methoxyphenol, due to + R effect of the – OCH3 group the electron density in the O – H bond increases thereby making the loss of proton difficult. Furthermore, the O-methoxyphenoxide ion left after the loss of a proton is destabilized by resonance because the two negative charges repel each other. So O-methoxyphenol is a weaker acid.
Question. Complete the following reaction equations

Answer.

Question. How will you convert the following?
(i) Propan-2-ol to propanone (ii) Phenol to 2, 4, 6-tribromophenol
Answer.

Question. How will you convert:
(i) Propene to Propane-1-ol? (ii) Ehtanal to Propan-2-ol?
Answer.
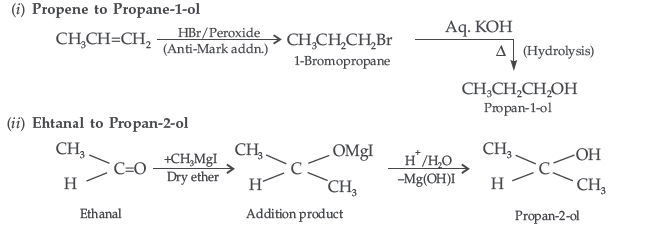
Question. Illustrate the following reactions giving a chemical equation for each :
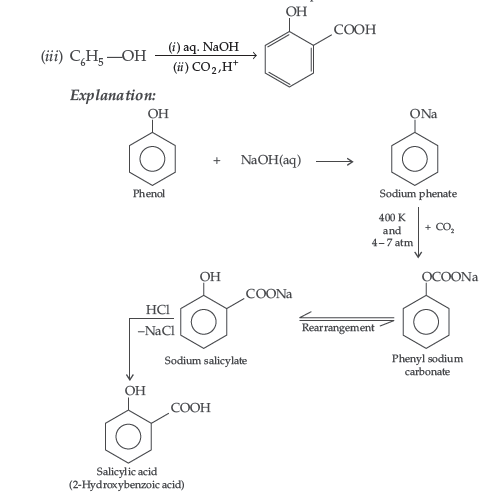
(i) Kolbe’s reaction (ii) Williamsons synthesis of an ether
Answer. (i) Kolbe’s reaction : Phenol reacts with CO2 in presence of sodium hydroxide (NaOH) at 4 – 7 Atm and 390 – 410 K giving salicylic acid
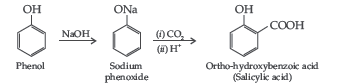
(ii) Williamsons synthesis of an ether : The reaction involves the nucleophilic substitution of the halide ion from the alkyl halide by the alkoxide ion by SN2 mechanism.
Example :
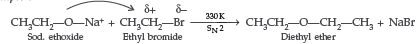
Question.47. Explain the mechanism of the following reaction:
Answer.
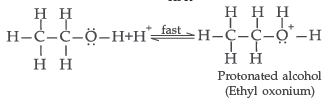
Question. Write the equations involved in the following reactions:
(i) Williamson ether synthesis (ii) Kolbe’s reaction
Answer.
(ii) Kolbe’s reaction : Phenoxide ion generated by treating phenol with sodium hydroxide is more reactive than phenol and undergoes electrophilic substitution with carbon dioxide. Ortho hydroxybenzoic acid is formed as the main reaction product.

Question. How are the following conversions carried out?
(i) Propene to propan-2-ol (ii) Ethylmagnesium chloride to propan-1-ol.
Answer. (i) Propene to propan-2-ol
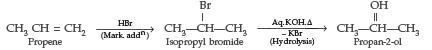
(ii) Ethylmagnesium chloride to propan-1-ol

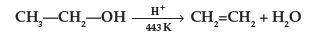
Question. Write the mechanism of acid dehydration of ethanol to yield ethene.
Answer. The mechanism of dehydration of ethanol involves the following steps :
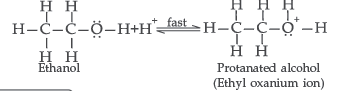
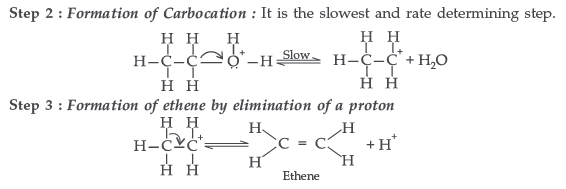
Question. How will you convert:
(i) Propene to propan-2-ol? (ii) Phenol to 2, 4, 6-trinitrophenol?
Answer.
(ii) Phenol to 2, 4, 6-trinitrophenol?

Question. Write the mechanism of the following reaction:
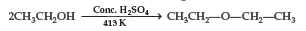
Answer. Mechanism:

Question. Explain the mechanism of dehydration steps of ethanol :
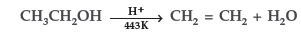
Answer.

Short Answer Type Questions-II
Question. Explain the following observations :
(i) The boiling point of ethanol is higher than that of methoxymethane.
(ii) Phenol is more acidic than ethanol.
(iii) o- and p-nitrophenols are more acidic than phenol.
Answer. (i) Due to presence of intermolecular H-bonding, associated molecules are formed, hence ethanol has high boiling point while methoxymethane does not have intermolecular H-bonding.
(ii) Phenol on losing H+ ion forms phenoxide ion, and ethanol on losing H+ ion forms ethoxide ion.Phenoxide ion is more stable than ethoxide ion as phenoxide ion exists in resonance structure. Due to this phenol is more acidic than ethanol.
(iii) Both o- and p-nitrophenols contain the NO2 group which is an electron withdrawing group. Due to –R and–I effect of the –NO2 group, electron density in the OH bond of substituted phenol decreases and hence the loss of proton becomes easy and therefore more acidic.
Question. Draw the structure and name the product formed if the following alcohols are oxidized. Assume that an excess of oxidising agent is used.
(i) CH3CH2CH2CH2OH (ii) 2-butanol (iii) 2-methyl-l-propanol
Answer.

Question. (a) How would you obtain the following :
(i) 2-methylpentan-2-ol from 2-methyl-1-pentene (ii) Acetophenone from phenol
(b) Write IUPAC name of the following :
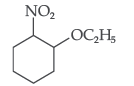
Answer.

Question. How would you obtain the following :
(i) Benzoquinone from phenol (ii) 2-Methylpropan-2-ol from ethylmagnesium chloride
(iii) Propan-2-ol from propene
Answer. (i) Benzoquinone from phenol
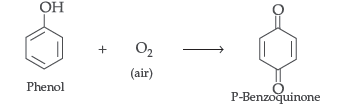
Question. Why phenol is more acidic than ethanol?
Answer. Phenol on losing H+ ion forms phenoxide ion, and ethanol on losing H+ ion forms ethoxide ion. Phenoxide ion is more stable than ethoxide ion as phenoxide ion exists in resonance structure. Due to this phenol is more acidic than ethanol.
Question. (a) Give a seperate chemical test to distinguish between the following pairs of compounds :
(i) Ethanol and Phenol (ii) 2-Pentanol and 3-Pentanol
(b) Explain Kolbe’s reaction with the help of suitable example.
Answer. (a) (i) Ethanol on reacting with I2 in NaOH gives yellow ppt of iodoform whereas phenol does not respond to this test.
(ii) 2-Pentanol on reacting with I2 in NaOH gives yellow ppt of iodoform whereas 3-pentanol does not respond to this test.
(b) Kolbe’s reaction : Phenol reacts with NaOH to give sodium phenoxide which on reaction with CO2 in acid gives salicylic acid.

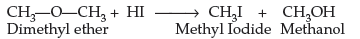
Question. (a) What happens when CH3—O—CH3 is heated with HI?
(b) Explain mechanism for hydration of acid catalyzed ethene :
Answer. (a) Methyl Iodide (CH3I) and Methanol (CH3OH) are formed when CH3—O—CH3 is heated with HI.
(b) Acid catalysed hydration : Alkenes react with water in the presence of acid as catalyst to form alcohols.
Mechanism : It involves the following three steps :

Question. (a) Give mechanism of preparation of ethoxy ethane from ethanol.
(b) How is toluene obtained from phenol?
Answer. (a) Mechanism of formation of ethoxy ethane

Question. (a) Illustrate the following name reactions :
(i) Reimer-Tiemann Reaction (ii) Williamson Synthesis.
(b) Give a chemical test to distinguish between 2-propanol and 2-methyl-2-propanol.
Answer.
(b) 2-Propanol is a secondary alcohol. When it reacts with I2 in NaOH, it forms a yellow ppt of iodoform but 2-methyl-2 propanol does not respond to this test.
Question. (a) Give chemical tests to distinguish between the following pairs of compounds :
(i) Pentan-2-ol and Pentan-3-ol (ii) Methanol and Phenol
(b) o-nitro phenol is more acidic than o-methoxy phenol. Explain why.
Answer. (a) (i) 2-pentanol gives Iodoform test with yellow ppt. of Iodoform while 3-pentanone does not give this test.
(ii) Distinction between Methanol and Phenol :
By FeCl3 test : Phenol gives violet coloured solution with FeCl3 while methanol does not.
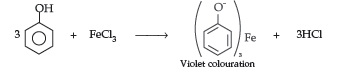
(b) O-nitro phenol is more acidic than o-methoxy phenol due to presence of NO2 group which has –I effect.It weakens the O – H bond of phenol by withdrawing their electrons and thus releases H+ ion easily while due to +I, +R effect of OCH3, o-methoxy phenol is less acidic.
Question. Write the major product in the following equations :
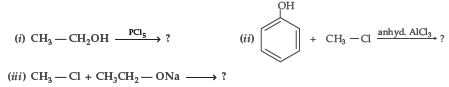
Answer.
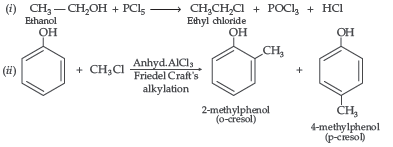
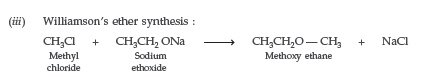
Question. Write the mechanism of the following reaction :

Answer.
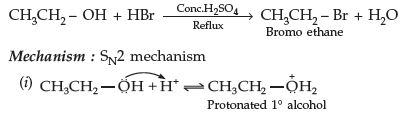
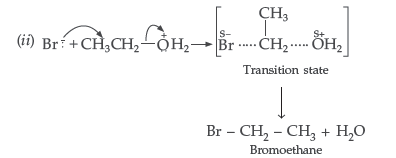
Question. How would you convert the following :
(i) Phenol to benzoquinone (ii) Propanone to 2-methylpropan-2-ol
(iii) Propene to propan-2-ol
Answer.

Question. How do you convert the following :
(i) Phenol to anisole (ii) Propan-2-ol to 2-methylpropan-2-ol (iii) Aniline to phenol (Delhi) Anisole
Answer.

Question. Explain the mechanism of the following reactions :
(i) Addition of Grignard’s reagent to the carbonyl group of a compound forming an adduct followed by hydrolysis.
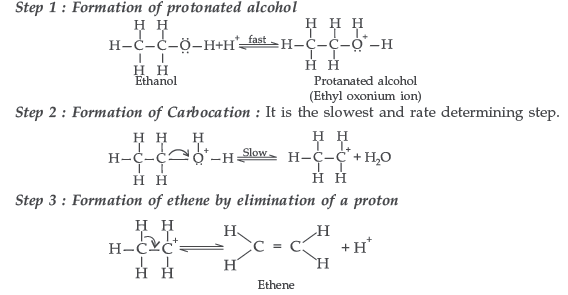
(ii) Acid catalysed dehydration of an alcohol forming an alkene.
(iii) Acid catalysed hydration of an alkene forming an alcohol.
Answer. (i) Carbonyl group undergoes nucleophillic addition reaction with Grignard reagent to form an adduct which undergoes hydrolysis to give alcohol in the following manner :
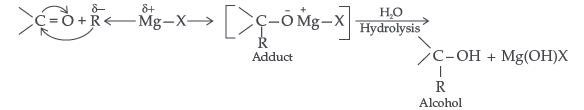
(ii) The mechanism of dehydration of ethanol involves the following steps :
Mechanism : It involves the following three steps :
(iii) Acid catalysed hydration : Alkenes react with water in the presence of acid as catalyst to form alcohols.
Mechanism : It involves the following three steps :
Step 1 : Protonation of alkene to form carbocation by electrophilic attack of H3O+
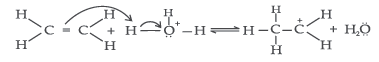
Question. Write the main product(s) in each of the following reactions:

Answer.

Question. Give reasons for the following :
(i) Phenol is more acidic than methanol.
(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (190°28′).
(iii) (CH3)3C—O—CH3 on reaction with HI gives (CH3)3C—I and CH3—OH as the main products and not (CH3)3C—OH and CH3—I.
Answer. (i) Phenol is more acidic than methanol because in phenol, phenoxide ion formed is more stabilized by resonance than phenol. There is no resonance in methanol.
(ii) The C—O—H bond angle in alcohols is slightly less than tetrahedral angle due to repulsion between the lone pairs of electrons of oxygen.
(iii) (CH3)3C+ is 3° carbo-cation which is more stable than CH3 + for SN1 reaction.
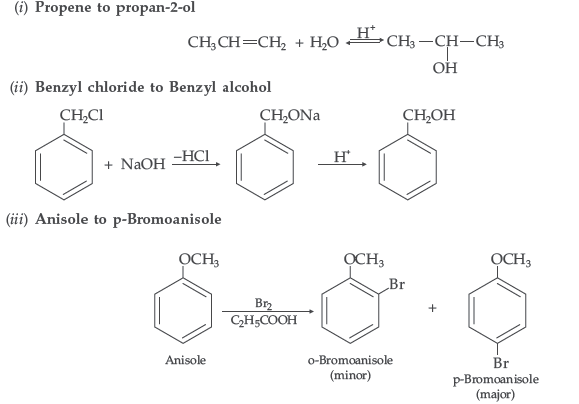
Question. How are the following conversions carried out?
(i) Propene to propan-2-ol (ii) Benzyl chloride to Benzyl alcohol
(iii) Anisole to p-Bromoanisole
Answer.
Question. How are the following conversions carried out?
(i) Propene → Propan-2-ol (ii) Ethylmagnesium chloride → Propan-1-ol
(iii) Benzyl chloride → Benzyl alcohol (Comptt All India)
Answer. (i) Propene to propan-2-ol
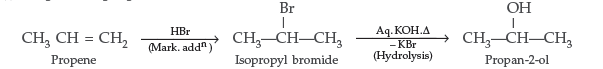
Question. Write the final product(s) in each of the following reactions:
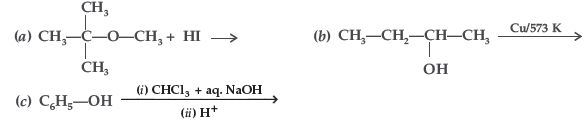
Answer.

Question. How are the following conversions carried out?
(i) Propene → Propan-2-ol (ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl magnesium chloride → Propan-1-ol
Answer.
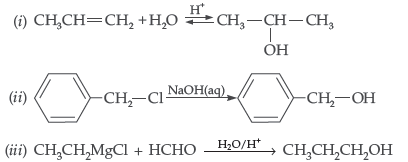
Question. Explain the following with an example in each :
(i) Kolbe’s reaction (ii) Reimer-Tiemann reaction
(iii) Williamson ether synthesis
Answer. (i) Kolbe’s reaction : Phenol reacts with CO2 at 390-400 K under pressure 4-7 giving Selicyle Acid
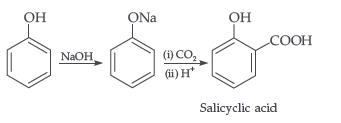
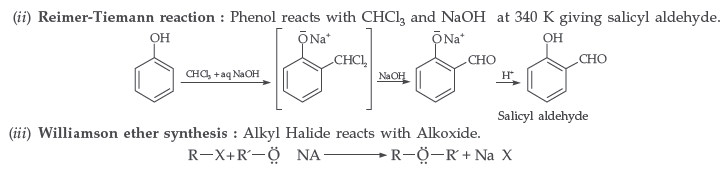
Question. (a) Write the mechanism of the following reaction :

(b) Write the equation involved in the acetylation of Salicylic acid.
Answer.

