Please refer to Class 10 Science Sample Paper Term 2 Set C with solutions below. The following CBSE Sample Paper for Class 10 Science has been prepared as per the latest pattern and examination guidelines issued by CBSE. By practicing the Science Sample Paper for Class 10 students will be able to improve their understanding of the subject and get more marks.
CBSE Class 10 Science Sample Paper for Term 2
SECTION – A
1. The atomic numbers of three elements A, B and C are 12, 18 and 20 respectively. Explain giving reason, which two elements will show similar properties.
Ans. Atomic number of A = 12
∴ Electronic configuration = 2, 8, 2
Similarly, for B(18) = 2, 8, 8
for C(20) = 2, 8, 8, 2
As elements A and C contain two valence electrons in their outermost shell (group-2), they will show similar properties.
2. How does the conductivity vary in diamond and graphite?
Ans. Diamond has no free electron hence, is a poor conductor of electricity whereas graphite has free electrons and hence is a good conductor of electricity.
3. What is the importance of DNA copying in reproduction?
Ans. DNA copying is important for reproduction as this process results in the production of offsprings which are genetically similar to parents. The exact blue print of body design is inherited to the offspring due to DNA copying from parent cell. Additional copies of DNA are made during replication. Moreover, minor alterations during the process of DNA copying lead to variations. Such variations are useful for the survival of species over time.
4. When observed through a magnifying glass, bread mould fungus first look like a white cottony mass and later on turns black. Explain.
Ans. The tiny spores of ‘bread mould’ (a fungus) are almost always present in the air. If we keep a moist slice of bread aside for a few days, then the spores of bread mould present in air settle on the moist bread and germinate to form new fungus. If we observe the surface of this bread slice through a magnifying glass, then bread mould first look like a white cottony mass covering the bread slice which later on turns black.
5. Why do all the gametes formed in human females have an X chromosome?
Ans. Genotype of human female is 44 + XX. Human female is homogametic. During meiosis, at the time of gamete formation, only one X chromosome enters each gamete. Hence, all female gametes have genotype (22 + X).
OR
Write the basic features of mechanism of inheritance.
Ans. Basic features of mechanism of inheritance are:
(i) Characters are controlled by genes and each gene controls one character.
(ii) Chromosomes are gene bearers and genes are basic unit of heredity.
(iii) One form of allele may be dominant on other, i.e., genes are allelic in nature.
(iv) The two forms of alleles separate at the time of gamete formation, i.e., they do not mix with each other.
(v) Two allelic forms of a gene are brought together in zygote.
6. (a) An electric kettle consumes 1 kW of electric power when operated at 220 V. A fuse wire of what rating must be used for it?
(b) How does the heat H produced by a current passing through a fixed resistance wire depend on the magnitude of current I ?
Ans. (a) : P = 1 kW = 1000 W, V = 220 V
∴ I = P / V = 1000 / 220 = 4.54 A
Rating of fuse wire must be higher than 4.54 A, so fuse wire of rating 5 A must be used.
(b) Heat (H ) produced is directly proportional to the square of the current (I) When resistance remains constant, i.e., H ∝ I 2.
OR
Several electric bulbs designed to be used on a 220 V electric supply line, are rated 10 W. How many lamps can be connected in parallel with each other across the two wires of 220 V line if the maximum allowable current is 6 A?
Ans. In a parallel combination, each bulb has the voltage equal to that of the main line, and the sum of the currents drawn by each bulb would be equal to the allowable current.
From the given data,
Current flowing through each bulb
= Power / Voltage = 10 W / 220 V = 1 / 22 A
Total maximum allowed current = 6 A
Number of bulbs which can be connected
= 6 A / 1/22 A = 22 x 6 = 132
7. Why should biodegradable and non-biodegradable wastes be discarded in two separate dustbins?
Ans. Biodegradable wastes are decomposed naturally by the action of microbes which degrade them to their simple constituents enabling their nutrients to recycle among the biotic and abiotic components of ecosystem. However, non-biodegradable wastes cannot be disposed off naturally since they cannot be decomposed by microbes. Such wastes are either recycled, incinerated or put in landfills, etc. As the disposal methods of the two types of wastes are different, it is advisable to discard the two types of waste in two separate dustbins.
OR
Why is improper disposal of waste a curse to environment?
Ans. Improper disposal of wastes is a curse to environment because of the following reasons:
(i) Wastes (both biodegradable and non-biodegradable) pollute the environment.
(ii) These have various harmful effects on living beings.
(iii) Biodegradable wastes pollute the environment only when their rate of accumulation is higher than their rate of degradation. On the other hand, nonbiodegradable wastes are not disposed off easily and keep on accumulating, causing serious harms to various organisms. Moreover, these wastes on burning release many toxic gases in the environment and if they accumulate on ground they cause soil and water pollution. Hence, there is no proper way of disposing nonbiodegradable wastes which is a curse to the environment.
SECTION – B
8. What are the main properties of covalent compounds with respect to melting point, boiling point and solubility ?
Ans. (i) Melting and boiling points : Since covalent compounds are made-up of electrically neutral molecules, therefore, the force of attraction between the molecules of a covalent compound is very weak. As a result, only small amount of heat energy is required to break these weak intermolecular forces. Therefore, covalent compounds have low melting and boiling points. For example, naphthalene has a low melting point of 353 K and carbon tetrachloride has a low boiling point of 350 K. However, there are some exceptions like diamond and graphite which are covalent solids and have very high melting and boiling points.
(ii) Solubility : Covalent compounds generally dissolve readily in organic solvents but much less in water. For example, naphthalene which is an organic compound dissolves readily in organic solvents like ether but is insoluble in water. However, some covalent compounds like urea, glucose, sugar, etc. are soluble in water. Some polar covalent compounds like ammonia and hydrochloric acid are also soluble in water.
9. How can it be proved that the basic structure of the Modern Periodic Table is based on the electronic configuration of atoms of different elements?
Ans. Electronic configuration of an element decides its position in Modern periodic table.
Lets take an example of sodium (Na).
Atomic number of sodium = 11
Thus, electronic configuration of Na = 2, 8, 1
As Na contains 1 electron in its outermost shell, it belongs to group 1. Sodium contains 3 shells so, it belongs to period number 3.
Thus, we can conclude that
Group number = Number of valence electrons
(When valence electrons are 1 and 2)
and group number = 10 + valence electrons
(When valence electrons are 3 and above)
Period number = Number of shells in which electrons are filled.
OR
How does the tendency of the elements to lose electrons change in the Modern Periodic Table in
(i) a group, (ii) a period and why?
Ans. (i) Tendency of the elements to lose electrons increases down the group. The reason being that at each succeeding element down a group, the number of shells increases. So, the distance of the valence shell from the nucleus increases due to which the effective nuclear charge decreases on the last shell of electrons. So, it becomes easier for the atom to lose electrons.
(ii) Tendency of the elements to lose electrons decreases in a period from left to right. The reason being that as the electron enters to the same shell at each successive element so, the effective nuclear charge on the valence shell electron increases, the attraction between the valence electrons and nucleus increases so, it becomes difficult to lose electrons.
10. How did Mendel explain that it is possible that a trait is inherited but not expressed in an organism ?
Ans. Mendel first selected two pureline plants having contrasting characters. He then crossed such plants. In the F1 generation, he observed that only one of the two contrasting characters appeared, he called it dominant and the one which does not get expressed in F1 was recessive. He later selfed the F1 plants and observed that both the traits appear but in a definite proportion. It can be explained by the following cross :

This is how Mendel explained that a trait may be inherited but not expressed in the plant.
11. (a) State the direction of magnetic field in the following case.

12. Free electrons always keep on moving in a conductor. Even then, no magnetic force acts on them in magnetic field unless a current is passed through it. Why?
Ans. In the absence of electric current, the free electrons in a conductor are in a state of random motion, like molecules in a gas. Their average velocity is zero i.e., they do not have any net velocity in a direction. As a result of it there is no net magnetic force on the free electrons, in the magnetic field. On passing the current, the free electrons acquire drift velocity in a definite direction, hence magnetic force acts on them in a magnetic field.
OR
A circular loop C and a solenoid AB carrying a current I each are shown below. Find the
(a) polarity of the loop face facing us,
(b) polarity of the end B of the solenoid and
(c) also find the direction of the magnetic field at the centre of the loop.
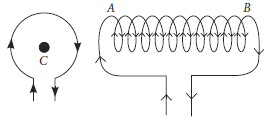
Ans. (a) We know if the current at a loop face facing us is in clockwise direction, that face of the coil behaves like South pole. So, the polarity of the loop facing us is South pole.
(b) The end B, when looked at from right carries a clockwise current. So, the polarity of the end B of the solenoid will be the South pole.
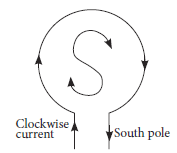
(c) By Fleming’s left-hand rule, the direction of the magnetic field at the centre of the loop will be perpendicular to the plane of the loop and will be directed inwards.
13. (a) Why should ozone layer be protected to save the environment?
(b) List any two ways which can help in protection of ozone layer as well as the environment.
Ans. (a) The ozone layer is very important for the existence of life on earth because it forms a protective shield around Earth by absorbing most of the harmful ultraviolet (UV) radiations. The UV radiations have extremely harmful effects on human beings, animals and plants as well, i.e., cause mutations, skin cancer, cataract, damage immune system, etc. So, ozone layer must be protected to save the environment.
(b) The two ways which can help in protection of ozone layer and environment are :
(i) Reduce the use of chemicals like chlorofluorocarbons (CFCs) which are widely used in refrigerators, air conditioners (as a coolant), in fire extinguishers and in aerosol sprayers. They destroy the ozone layer gradually. We can protect our ozone layer by avoiding the use of such objects which releases CFCs.
(ii) Nitrous oxide is the largest ozone depleting substance as well as greenhouse gas released by human activities, such as from motor vehicles, fertilisers. People should be encouraged to use more public transport, car pooling, using hybrid or electric cars and use of fertiliser formulations to reduce emission of nitrous oxide.
SECTION – C
This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (a, b and c). Parts a and b are compulsory. However, an internal choice has been provided in part c.
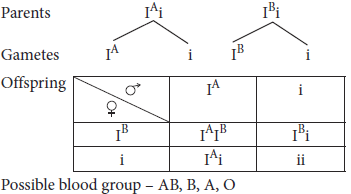
14. A man with blood group A marries a woman with blood group B. They had 2 children, 1 with AB blood group and other with blood group B.
(a) What could be the genotype of parents?
(b) Briefly discuss multiple allelism.
(c) What all blood group are possible in offspring in the given condition?
Ans. (a) Possible genotypes of parents are – IAi and IBi.
(b) Multiple alleles are three or more alternative forms of a gene that can occupy the same locus but only two of the alleles can be present in single organism.
(c) Cross between man with blood group A and woman with blood group B would result in following blood groups in progeny:
OR
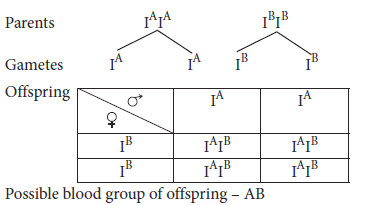
If both parents are homozygous for both blood groups A and B, then what would be the blood group of offspring?
Ans.
15. For a series combination of resistors, Rs = ΣRi and current through each resistor is same. In a parallel combination of resistors, 1 / Rp = 1/ R1 + 1 / R2 +…….and potential drop across each resistor is same.
(a) If we connect n bulbs each with a rated power P in series, then find the total power consumed by combination at rated current.
(b) If we connect n bulbs each with a rated power P in parallel, then find the total power consumed by combination at rated voltage.
(c) The power consumed by n equal resistance in parallel is x times that of power consumed in series if the voltage supply is same. Then what will be value of x ?
Ans.

OR
How is the current conducted in metals? Explain.
Ans. Every metallic conductor has large number of free electrons which move randomly at room temperature. Their average thermal velocity at any instant is zero. When a battery or cell is concentrated across the ends of a conductor, the free electrons of the conductor experience force and drift towards the positive end of the conductor, causing an electric current (i.e. conduction current) in the conductor whose direction is opposite to the direction of motion of the free electrons in the conductor.

