Please see Chapter 3 Electrochemistry Exam Questions Class 12 Chemistry below. These important questions with solutions have been prepared based on the latest examination guidelines and syllabus issued by CBSE, NCERT, and KVS. We have provided Class 12 Chemistry Questions and answers for all chapters in your NCERT Book for Class 12 Chemistry. These solved problems for Electrochemistry in Class 12 Chemistry will help you to score more marks in upcoming examinations.
Exam Questions Chapter 3 Electrochemistry Class 12 Chemistry
Short Answer Type Questions-I
Question. The resistivity of a 0.8M solution of electrolyte is 5 × 10–3 Ωcm. Calculate its molar conductivity.
Answer. Resistivity (ρ) = 5 × 10–3 Ω cm
Conductivity of solution(k)
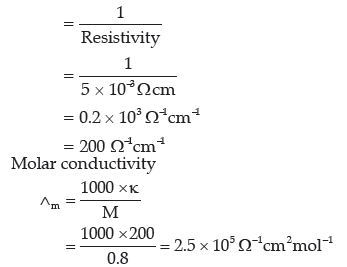
Question. Why on dilution the Lm of CH3COOH increases drastically, while that of CH3COONa increases gradually?
Answer. In case of CH3COOH which is a weak electrolyte, the number of ions increases on dilution due to an increase in degree of dissociation resulting in drastic increase in Lm.
CH3COOH+H2O CH3COO− +H3O +
In the case of CH3COONa which is a strong electrolyte, the number of ions remains the same but the inter-ionic attraction decreases resulting in gradual increase in Lm.
Question. From the given cells:
Lead storage cell, Mercury cell, Fuel cell and Dry cell.
Answer the following:
(i) Which cell is used in hearing aids?
(ii) Which cell was used in Apollo Space Programme?
(iii) Which cell is used in automobiles and inverters?
(iv) Which cell does not have long life?
Answer. (i) Mercury cell
(ii) Fuel cell
(iii) Lead storage cell
(iv) Dry cell
Question. Write the name of the cell which is generally used in transistors. Write the reactions taking place at the anode and the cathode of this cell.
Answer. Dry cell/Leclanche cell
Anode: Zn(s) → Zn2+ + 2e–
Cathode: MnO2 + NH4+ + e– → MnO(OH) + NH3
Question. Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The limiting molar conductivity of ‘B’ increases 1.5 times while that of ‘A’ increases 25 times. Which of the two is a strong electrolyte ? Justify your answer.
Answer. ‘B’ is a strong electrolyte. B is a strong electrolyte which is completely dissociated into ions, but on dilution interionic forces overcome and ions are free to move. So there is slight increase in molar conductivity on dilution.
Question. Write the electrode reactions for a H2–O2 fuel cell.
Answer.

Question. In a galvanic cell, the following cell reaction occurs:
Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s)
Eocell = +1.56 V
(i) Is the direction of flow of electrons from zinc to silver or silver to zinc?
(ii) How will concentration of Zn2+ ions and Ag+ ions be affected when the cell functions?
Answer. (i) Zinc to silver
(ii) Concentration of Zn2+ ions will increase and Ag+ ions will decrease.
Question. Calculate the degree of dissociation (a) of acetic acid if its molar conductivity (Λm) is
39.05 S cm2 mol-1. Given Λ˚(H+) = 349.6 S cm2 mol-1 and Λ°(CH3COO–) = 40.9 S cm2 mol-1.
Answer. Λ°CH3COOH = Λ°CH3COO– + Λ°H+
= 40.9 + 349.6 = 390.5 S cm2/mol
Now, α = Λm/Λ°m
= 39.05/390.5 = 0.1
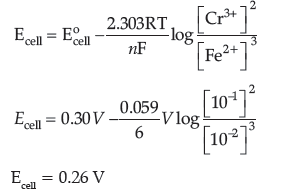
Question. Calculate the emf of the following cell at 298 K Cr(s)|Cr3+ (0.1M)||Fe2+ (0.01M)|Fe(s)
[Given: E°cell = + 0.30 V]
Answer. 2Cr(s) + 3 Fe2+(aq) → 3Fe(s) + 2Cr3+ (aq)
Where, n = 6
Question. Following reactions can occur at cathode during the electrolysis of aqueous silver nitrate solution using Pt electrodes:

On the basis of their standard electrode potential values, which reaction is feasible at cathode and why ?
Answer. Ag+(aq) + e− → Ag(s)
Because it has higher reduction potential.
Detailed Answer: As reaction with higher value of standard electrode potential occurs at cathode, Ag gets reduced. So, the reaction occurring at cathode is
Ag+(aq) + e− → Ag(s)
Question. The conductivity of a 0.01 M solution of acetic acid at 298 K is 1.65 × 10–4 S cm–1.
Calculate molar conductivity (Λm) of the solution.
Answer.
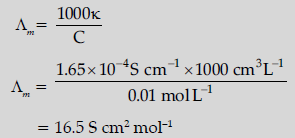
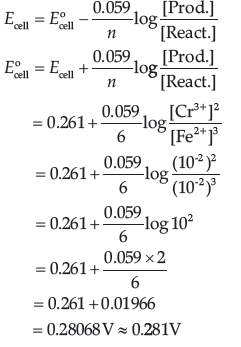
Question. Calculate E°cell for the following reaction at 298 K:
2Cr(s) + 3Fe2+ (0.01M) → 2Cr3+(0.01M) + 3Fe(s)
Given: Ecell = 0.261 V
Answer. Nernst Equation:
Question. The conductivity of 10-3 mol/L acetic acid at 25°C is 4.1 × 10–5 S cm–1. Calculate its degree of dissociation if Λ°m for acetic acid at 25°C is 390.5 S cm2 mol-1.
Answer.
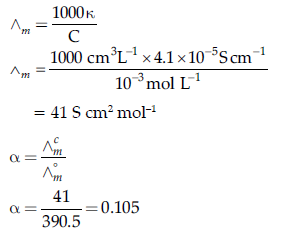
Question. Write the name of the cell which is generally used in hearing aids. Write the reactions taking place at the anode and the cathode of this cell.
Answer. Mercury cell
Anode: Zn(Hg) + 2OH– → ZnO(s) + H2O + 2e–
Cathode: HgO + H2O + 2e– → Hg(l) + 2OH–
Question. Explain redox potential.
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.
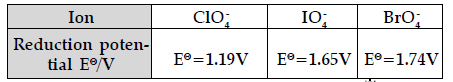
Answer. Redox potential (also known as reduction / oxidation potential) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is measured in volts (V), or millivolts (mV). The more positive the reduction potential of a species, the greater the species’ affinity for electrons and tendency to be reduced. The higher the reduction potential, the higher is its tendency to get reduced. Hence, the order of oxidising power is:
BrO4–> IO4– > ClO4–
Question. Iron displaces copper from copper sulphate solution but Pt does not why?
Answer. Electrode potential of Fe is more than electrode potential of Cu. So, Fe displaces Cu from copper sulphate while electrode potential of Pt is less than Cu. Due to this reason, Pt cannot displace Cu from copper sulphate.
Short Answer Type Questions-II
Question. (a) The cell in which the following reaction occurs:
2 Fe3+ (aq) + 2 I– (aq) → 2 Fe2+ (aq) + I2 (s)
has E°cell = 0.236 V at 298 K. Calculate the standard Gibb’s energy of the cell reaction.
(Given: 1 F = 96,500 C mol–1)
(b) How many electrons flow through a metallic wire if a current of 0.5 A is passed for 2 hours ?
(Given: 1 F = 96,500 C mol–1)
Answer. (a) ΔG° = – nFE°cell
n = 2
ΔG° = – 2 × 96500 C /mol × 0.236 V
= – 45548 J/mol
= – 45.548 kJ/mol
(b) Q = I t = 0.5 × 2 × 60 × 60
= 3600 C
96500 C = 6.023 × 1023 electrons
3600 C = 2.25 × 1022 electrons
Question. The electrical resistance of a column of 0.05 M KOH solution of diameter 1 cm and length 45.5 cm is 4.55 × 103 ohm. Calculate its molar conductivity.
Answer. A = pr2
= 3.14 × 0.5 × 0.5 cm2
= 0.785 cm2
l = 45.5 cm
G* = l/A = 45.5 cm/0.785 cm2
= 57.96 cm–1
k = G*/R
= 57.96 cm–1/4.55 × 103 Ω
= 1.27 × 10–2 S cm–1
∧m = k × 1000/C
= [1.27 × 10–2 S cm–1] × 1000/0.05 mol/cm3
= 254.77 S cm2 mol–1
Question. The electrolysis of a metal salt solution was carried out by passing a current of 4 A for 45 minutes.
It resulted in deposition of 2.977 g of a metal. If atomic mass of the metal in 106.4 g mol–1, calculate the charge on the metal cation.
Answer. Let the charge on the metal ion = n+
Reduction half-reaction,
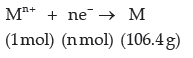
Quantity of electricity required for depositing 106.4 g of metal = n × 96500 C
Quantity of electricity required for depositing 2.977 g of metal = (n ×96500× 2.977)/106.4=n × 2700
Quantity of electricity passed
= 4 × 45 × 60 C
= 10800 C
Applying law of conservation of charge,
10800 = n × 2700
n = 10800/2700 = 4
Charge on metal ion = +4
Question. Consider the following reaction:
Cu(s) + 2Ag+(aq) → 2Ag(s) + Cu2+(aq)
(i) Depict the galvanic cell in which the given reaction takes place.
(ii) Give the direction of flow of current.
(iii) Write the half-cell reactions taking place at cathode and anode.
Answer. (i) Cu(s) | Cu2+(aq) || Ag+ (aq) | Ag(s)
(ii) Current will flow from silver to copper electrode in the external circuit.
(iii) Cathode: 2Ag+(aq) + 2e– → 2Ag(s)
Anode: Cu(s) → Cu2+ (aq) + 2e–
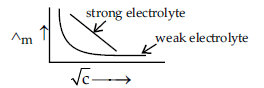
Question. The following curve is obtained when molar conductivity (Λm) is plotted against the square root of concentration, C1/2 for two electrolytes A and B:
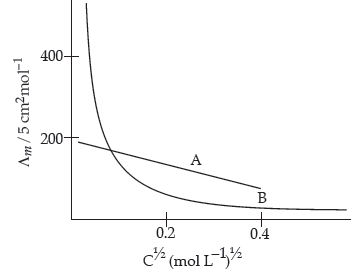
(i) How do you account for the increase in the molar conductivity of the electrolyte A on dilution ?
(ii) As seen from the graph, the value of limiting molar conductivity (Λ°m) for electrolyte B cannot be obtained graphically. How can this value be obtained ?
(iii) Define limiting molar conductivity.
Answer. (i) As seen from the graph, electrolyte A is a strong electrolyte which is completely ionised in solution. With dilution, the ions are far apart from each other and hence the molar conductivity increases.
(ii) To determine the value of limiting molar conductivity for electrolyte B, indirect method based upon Kohlrausch’s law of independent migration of ions is used.
(iii) When concentration approaches zero, the molar conductivity is known as limiting molar conductivity.
Question. Calculate ΔrG° and log Kc for the following reaction at 298 K.
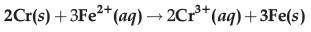
[(E°cell = 0.30 V), 1F = 96500 C mol-1]
Answer. ΔrG° = -nFE°cell, n = 6
= -6 × 96500 C/mol × 0.30 V
= -173700 J/mol = -173.7 kJ/mol
E°cell = 0.059V/n × log Kc
log Kc = 0.30 V × 6/0.059 V = 30.5
Question. (i) State the law which helps to determine the limiting molar conductivity of weak electrolyte.
(ii) Calculate limiting molar conductivity of CaSO4 (limiting molar conductivity of calcium and sulphate ions are 119.0 and 160.0 S cm2 mol–1 respectively)
Answer. Kohlrausch law of independent migration of ions:
(i) The limiting molar conductivity of an electrolyte can be represented as the sum of the individual contribution of the anions and cations of the electrolyte.
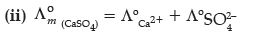
= 119.0 S cm2 mol–1 + 160.0 S cm2 mol–1
= 279.0 S cm2 mol–1
Question. Calculate e.m.f. of the following cell at 298 K:
2Cr(s) + 3Fe2+ (0.1M) → 2Cr3+ (0.01M) + 3Fe(s)
E°(Cr3+ / Cr) = – 0.74 V
E° (Fe2+ / Fe) = – 0.44 V.
Answer.

Question. A galvanic cell consists of a metallic zinc plate immersed in 0.1 M Zn(NO3)2 solution and metallic plate of lead in 0.02 M Pb(NO3)2 solution. Calculate the emf of the cell. Write the chemical equation for the electrode reactions and represent the cell.
(Given: E°Zn2+/Zn = – 0.76 V; E°Pb2+/Pb = – 0.13V)
Answer. Anode reaction: Zn(s) → Zn2+(aq) + 2e–
Cathode reaction: Pb2+(aq) + 2e– → Pb(s)
Cell representation:
Zn(s)|Zn2+(aq)||Pb2+(aq)|Pb(s)
According to Nernst equation:
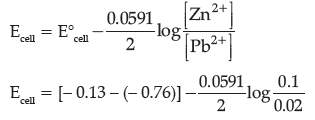
= 0.63 – 0.02955 × log 5
= 0.63 – 0.02955 × 0.6990
= 0.63 – 0.0206 = 0.6094
Question. Calculate the emf of the following cell at 25° C:
Fe | Fe2+ (0.001 M) || H+ (0.01 M) | H2(g) (1bar) | Pt(s)
E° (Fe2+ / Fe) = – 0.44 V E° (H+ / H2) = 0.00 V
Answer. Cell reaction is
Fe(s) + 2H+(aq) → Fe2+(aq) + H2(g)
E°cell = 0.00 – (– 0.44) = 0.44 V

Question. When a steady current of 2A was passed through two electrolytic cells A and B containing electrolytes ZnSO4 and CuSO4 connected in series, 2 g of Cu were deposited at the cathode of cell B.How long did the current flow? What mass of Zn was deposited at cathode of cell A?
[Atomic mass: Cu = 63.5 g mol–1, Zn = 65 g mol–1; 1F = 96500 C mol–1]
Answer.
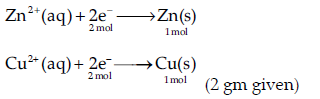
The charge Q on a mole of electrons, Q = nF
Calculation of time for the flow of current:
n = 1 mol
Q = 1 × 96500 C mol–1 = 96500 C
Molar mass of Cu = 63.5 gm mol–1
∵ 63.5 gm of Cu is deposited by electric charge = 96500C
∴ 2 gm of Cu is deposited by electric charge = (96500 /63.5)×2 = 3039.37 C
Let 2 A of current be passed for time t, quantity of electricity used = 2A × t = 3039.37 C
or, t = 3039.37C/2 = 1519.68 s
= 25 min 33 s
Question. (i) Give Debye Huckel Onsager equation for strong electrolyte.
(ii) Given are the conductivity and molar conductivity of NaCl solutions at 298K at different concentrations:
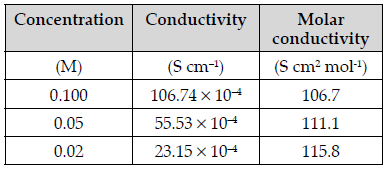
Compare the variation of conductivity and molar conductivity of NaCl solutions on dilution. Give reason.
Answer. (i) Debye Huckel Onsager equation for strong electrolyte is:
∧m =∧∞m −b√ C
Where ∧m = Molar conductivity
∧∞m = m Molar conductivity at infinite dilution
b = Constant
C = Concentration of solution
(ii) Conductivity of NaCl decreases on dilution as the number of ions per unit volume decreases.
Whereas molar conductivity of NaCl increases on dilution as on dilution the interionic interactions overcome and ions are free to move.
Question. (a) Calculate the mass of Ag deposited at cathode when a current of 2 amperes was passed through a solution of AgNO3 for 15 minutes.
(Given: Molar mass of Ag = 108 g mol–1,1 F = 96500 C mol–1)
(b) Define fuel cell.
Answer.
(a) m = ZI
= (108× 2× 15× 60)/1× 96500
= 2.01 g (or any other correct method)
(b) Cells that convert the energy of combustion of fuels directly into electrical energy.
Long Answer Type Questions
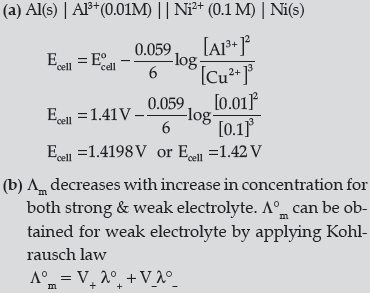
Question. (a) Represent the cell in which the following reaction takes place:
2Al( s)+ 3Ni2+ ( 0.1M) → 2Al3+ ( 0.01M)+ 3Ni( s) Calculate its emf if Eocell = 1.41V.
(b) How does molar conductivity vary with increase in concentration for strong electrolyte and weak electrolyte? How can you obtain limiting molar conductivity ( ∧om ) for weak electrolyte?
Answer.
Question. (a) The conductivity of 0.001 mol L–1 acetic acid is 4.95 × 10–5 S cm–1. Calculate the dissociation constant if ∧om for acetic acid is 390.5 S cm2 mol–1.
(b) Write Nernst equation for the reaction at 25°C:
2Al(S)+ 3Cu2+ (aq) → 2Al3+ (aq)+ 3Cu(s)
(c) What are secondary batteries? Give an example.
Answer.
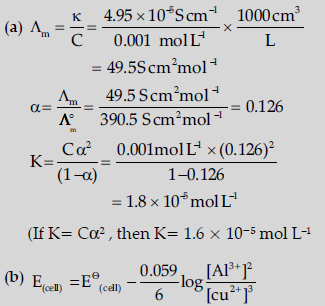
(c) Batteries which are rechargeable
Example- Lead storage, Ni-Cd batteries (Or any other one example).
Question. (a) Calculate e.m.f. of the following cell:
Zn(s)/Zn2+ (0.1 M) || (0.01 M) Ag+/Ag(s)
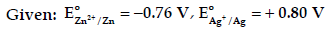
[Given: log 10 = 1]
(b) X and Y are two electrolytes. On dilution molar conductivity of ‘X’ increases 2.5 times while that Y increases 25 times. Which of the two is a weak electrolyte and why?
Answer. (a) Zn(s)/ Zn2+ (0.1 M) || (0.01M) Ag+/Ag(s)
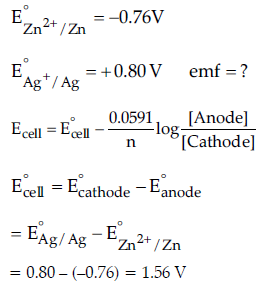
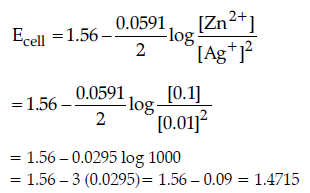
(b) Y is a weak electrolyte as on dilution complete dissociation of weak electrolyte takes place and thus a sharp increase in molar conductivity while in strong electrolyte it has already dissociated completely. So on dilution molar conductivity does not rises much.
Question. (a) For the reaction
2AgCl (s) + H2 (g) (1 atm) → 2Ag(s)+2H+ (0.1 M)+2Cl–(0.1 M),
ΔG°= – 43600 J at 25°C.
Calculate the e.m.f. of the cell.
[log 10–n = –n]
(b) Define fuel cell and write its two advantages.
Answer. (a) ΔGo = – nFEo
–43600 = – 2 × 96500 ×Eo
Eo = 0.226 V
E = Eo – 0.059/2 log ([H+]2 [Cl–]2 / [H2])
= 0.226 – 0.059/2 log[ (0.1)2 × (0.1)2 ] / 1
= 0.226 – 0.059 /2 log 10-4
= 0.226 + 0.118 = 0.344 V
(Deduct half mark if unit is wrong or not written)
(b) Cells that convert the energy of combustion of fuels (like hydrogen, methane, methanol etc.) directly into electrical energy are called fuel cells.
Advantages: High efficiency, non polluting (or any other suitable advantage)
Question. (a) The conductivity of 0.001 mol L–1 solution of CH3COOH is 3.905 × 10–5 S cm–1. Calculate its molar conductivity and degree of dissociation (α).
Given λ° (H+) = 349.6 S cm2 mol–1 and λ0 (CH3COO–) = 40.9 S cm2 mol–1
(b) Define electrochemical cell. What happens if external potential applied becomes greater than E°cell of electrochemical cell ?
Answer.


(b) Device used for the production of electricity from energy released during spontaneous chemical reaction and the use of electrical energy to bring about a chemical change.
The reaction gets reversed / It starts acting as an electrolytic cell & vice – versa.
Question. Eocell for the given redox reaction is 2.71 V.
Mg + Cu2+(0.01 M) → Mg2+ (0.001 M) + Cu
Calculate Ecell for the reaction. Write the direction of flow of current when an external opposite potential applied is:
(i) Less than 2.71 V
(ii) Greater than 2.71 V
Answer.
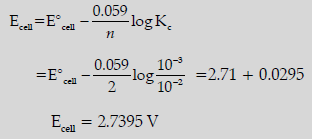
(i) Cu to Mg / Cathode to anode / Same direction
(ii) Mg to Cu / Anode to cathode / Opposite direction
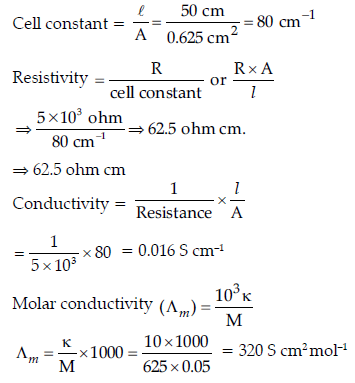
Question. (a) The electrical resistance of a column of 0.05 M KOH solution of length 50 cm and area of cross-section 0.625 cm2 is 5 × 103 ohm.
Calculate its resistivity, conductivity and molar conductivity.
(b) Predict the products of electrolysis of an aqueous solution of CuCl2 with platinum electrodes.

Answer. (a) Given: A = 0.625 cm2, l = 50 cm
R = 5 × 103 ohm, r = ?
m = 0.05 m, k = ?
∧m = ?
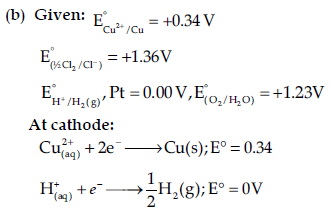
The reaction with a higher value of E° takes place at the cathode, so deposition of copper will take place at the cathode.
At anode: The oxidation reactions are possible at the anode.
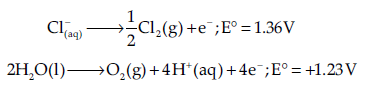
At the anode the reaction with a lower value of E° is preferred. But due to the over potential of oxygen, Cl– gets oxidised at anode to produce Cl2 gas.
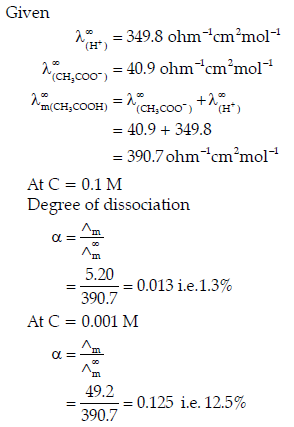
Question. The molar conductivities of acetic acid at 298 K at the concentrations of 0.1 M and 0.001 M are 5.20 and 49.2 S cm2mol-1 respectively. Calculate the degree of dissociation of acetic acid at these concentration. Given λ+(H+) and λ∞(CH3COO−) are 349.8 and 40.9 ohm-1cm2mol-1 respectively.
Answer.
Question. (a) Calculate E°cell for the following reaction at 298K:
2Al(s) + 3Cu2+ (0.01M) → 2Al3+ (0.01M) + 3Cu(s)
Given: E°cell = 1.98 V
(b) Using the E° values of A and B, predict which is better for coating the surface of iron
[E°(Fe2+/Fe) = – 0.44V] to prevent corrosion and why ?
Given: E°(A2+/A) = – 2.37V: E°(B2+/B) = – 0.14V
Answer.
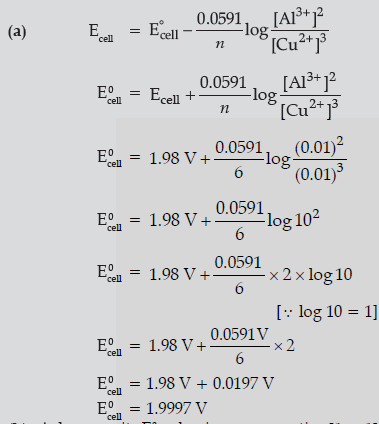
(b) A, because its E0 value is more negative
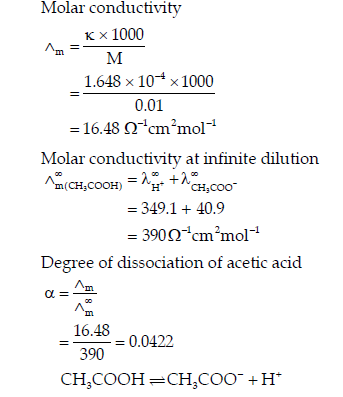
Question. The resistance of 0.01 M acetic solution when measured in a conductivity cell of cell constant 0.366 cm-1, is found to be 2220 Ω. Calculate degree of dissociation of acetic acid at this concentration.
Also find the dissociation constant of acetic acid.
Given that value of λ+(H+) and λ∞(CH3COO−) 40.9 Ω-1cm2mol-1 respectively.
Answer. Conductivity(k) of 0.01 M acetic acid
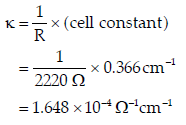

Question. (a) A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4 and ZnSO4 until 2.8 g of Fe deposited at the cathode of cell X.
How long did the current flow? Calculate the mass (Molar mass: Fe = 56 g mol-1, Zn = 65.3 g mol-1, 1 F = 96500 C mol-1)
(b) In the plot of molar conductivity Λm vs. square root of concentration (C½), following curve obtained for two electrolytes A and B:

Answer the following:
(i) Predict the nature of electrolytes A and B:
(ii) What happens on extrapolation of Λm to concentration approaching zero for electrolytes A and B?
Answer.
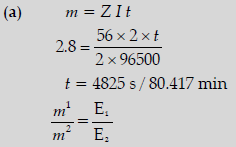

Question. (a) Write the cell reaction and calculate the e.m.f. of the following cell at 298 K:
Sn (s) | Sn2+ (0.004 M) || H+ (0.020 M) | H2 (g)(1 bar) | Pt (s)
(Given: E° Sn2+/ Sn = – 0.14V)
(b) Give reasons:
(i) On the basis of E° values, O2 gas should be liberated at anode but it is Cl2 gas which is liberated in the electrolysis of aqueous NaCl.
(ii) Conductivity of CH3COOH decreases on dilution.
Answer.
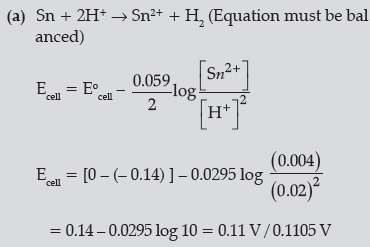
(b) (i) Due to over potential/ over voltage of O2
(ii) The number of ions per unit volume decreases.

