Please refer to Class 10 Science Sample Paper Term 1 Set F with solutions below. The following CBSE Sample Paper for Class 10 Science has been prepared as per the latest pattern and examination guidelines issued by CBSE. By practicing the Science Sample Paper for Class 10 students will be able to improve their understanding of the subject and get more marks.
CBSE Class 10 Science Sample Paper for Term 1
SECTION–A
1. Which of the following changes are exothermic in nature?
(a) Dissolution of ammonium chloride in water.
(b) Decomposition of silver bromide.
(c) Decomposition of ferrous sulphate.
(d) Dilution of sulphuric acid.
Answer
D
2. A dilute ferrous sulphate solution was added gradually to the beaker containing acidified potassium permanganate solution. The light purple colour of the solution fades and finally disappears. Select the correct statement.
(a) Colour disappears due to dilution as no reaction is involved.
(b) KMnO4 is oxidising agent and oxidises FeSO4.
(c) KMnO4 decomposes in presence of FeSO4 as KMnO4 is less stable.
(d) FeSO4 acts as oxidising agent and oxidises KMnO4
Answer
B
3. Balance the following chemical equation:
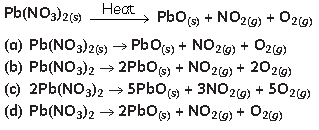
Answer
A
4. Why does not a wall immediately acquire a white colour when a coating of slaked lime is applied on it?
(a) slaked lime reacts with oxygen to form calcium carbonate, which imparts white colour
(b) slaked lime reacts with carbon dioxide to form calcium hydroxide which imparts white colour
(c) slaked lime reacts with carbon dioxide to form calcium carbonate which imparts white colour
(d) slaked lime turns white on solidification
Answer
C
5. The pH value of which of the salts will be greater than 7?
(I) Sodium Carbonate
(II) Sodium Chloride
(III) Sodium Sulphate
(IV) Sodium Hydrogen Carbonate
(a) Both (I) and (II)
(b) Both (II) and (IV)
(c) Both (I) and (III)
(d) Both (I) and (IV)
Answer
D
6. The process of dilution of acid or base with water will result in:
(a) No change in the concentration of ions (H3O+/OH–) per unit volume
(b) Decrease in the concentration of ions (H3O+/OH–) per unit volume
(c) Increase in the concentration of ions (H3O+/OH–) per unit volume
(d) Decrease in the concentration of H3O+ ions but increase in concentration of OH–ions per unit volume
Answer
A
7. Solution of a substance X changes its colour to pink when Phenolphthalein is added to it. Solution of another substance Y changes its colour to yellow on adding methyl orange as shown in figure below:
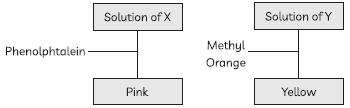
Identify the correct nature of solutions of X and Y:
(a) Both X and Y are basic
(b) X is basic and Y is acidic
(c) X is acidic and Y is basic
(d) Both X and Y are acidic
Answer
A
8. Which is the strong acid?
H2CO3, HNO3, HCl, CH3COOH
(a) HNO3, H2CO3
(b) HNO3, HCl
(c) HCl, CH3COOH
(d) H2CO3, CH3COOH
Answer
B
9. Why is sodium metal never left open in air?
(a) It melts at room temperature
(b) It reacts with moisture present in air violently
(c) It reacts with oxygen present in air violently
(d) Both (b) and (c)
Answer
C
10. Study the given table:
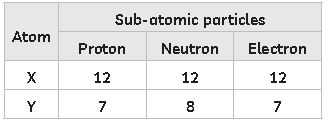
X and Y combine to form a compound of for mula:
(a) X3Y2
(b) X2Y3
(c) XY
(d) X3Y
Answer
A
11. The figure below shows the movement of water in a tree.

Based on the above figure, select the correct options:
(I) The loss of water in the form of vapour from the aerial parts of the plant is known as transpiration.
(II) Evaporation of water molecules from the cells of a leaf creates a suction which pulls water from the phloem cells of roots.
(III) Transpiration helps in the absorption and upward movement of water and minerals dissolved in it from roots to the leaves.
(IV) Transpiration is the major driving force in the movement of water in the xylem during night.
(a) Both (I) and (II)
(b) Both (II) and (III)
(c) Both (I) and (III)
(d) Both (III) and (IV)
Answer
C
12. Oxygen is transported by respiratory pigment as:
(a) It is more soluble in water than CO2
(b) Haemoglobin has high affinity for it.
(c) It easily diffuses to all body parts
(d) It is easily absorbed by our tissues..
Answer
B
13. Observe the diagram showing nutrition in Amoeba, where two parts have been labelled as (I) and (II), and select the correct option:
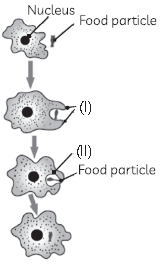
(a) The part labelled (I) is the food vacuole and stores the food
(b) The part labelled (I) is the pseudopodia and stores the food
(c) The part labelled (II) is the food vacuole and digests the food
(d) The part labelled (II) is the pseudopodia and digests the food.
Answer
C
14. Which enzyme is secreted by the gastric glands?
(a) Amylase
(b) Pepsin
(c) Trypsin
(d) Lipase
Answer
B
15. Which of the following is not a requisite of any respiratory surface to become highly efficient are:
(a) Large surface area
(b) Thick walls
(c) Rich blood supply
(d) Fine and delicate surface area
Answer
B
16. A part of the human respiratory system has been shown separately in a box with two parts labelled as X and Y.
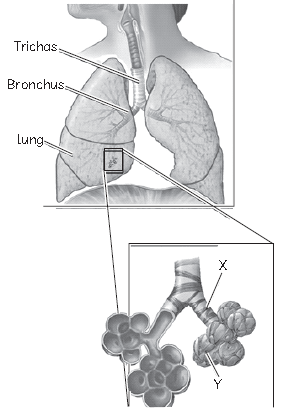
Identify the parts labelled X and Y from the table given below:
Answer
B
17. If a graph is plotted between sin r taken on X-axis and sin i taken on Y-axis, where i is the angle of incidence and r is the angle of refraction for a given pair of media, then:
(I) Graph will be a parabola
(II) Graph will be a straight line
(III) Slope of graph will give the refractive index of the second medium with respect to the first
(IV) Slope of graph will give the speed of light in the second medium.
(a) Both (I) and (III)
(b) Both (II) and (III)
(c) Both (I) and (IV)
(d) Both (II) and (IV)
Answer
B
18. Four students plotted the path of light through a rectangular glass slab.

The correct ray diagram has been plotted by:
(a) Student A
(b) Student B
(c) Student C
(d) Student D
Answer
C
19. A real image 1/5 th the size of the object is formed at a distance of 18 cm from a lens.
From the table select the row containing the correct identification of lens and focal length of the lens.

Answer
D
20. Examine the figure given below and select the correct option regarding magnification m produced by the concave mirror for the position of object AB as shown:
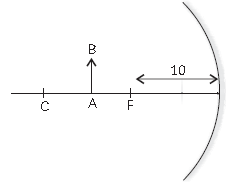
(a) m > 1
(b) m < 1
(c) m > – 1
(d) m < – 1
Answer
C
21. Harsh wants to see a magnified image while shaving in a mirror. What type of mirror should use:
(a) convex mirror
(b) concave mirror
(c) plane mirror
(d) combination of (a) and (b)
Answer
B
22. It the image formed by a spherical mirror for all positions of the object placed in front of it always erect and diminished what type of mirror is it:
(a) convex mirror
(b) plane mirror
(c) concave mirror
(d) could be concave and convex
Answer
A
23. The time difference between actual sunset and the apparent sunset is about:
(a) 2 min
(b) 3 min
(c) 4 min
(d) 5 min
Answer
A
24. What is the colour of sunlight scattered by the dust particles in the atmosphere:
(a) Blue
(b) Red
(c) White
(d) Violet
Answer
C
SECTION–B
25. Select the correct equation(s) which represent double displacement reaction?
(I) Pb + CuCl2 → PbCl2 + Cu
(II) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(III) C + O2 → CO2
(IV) CH4 + 2O2 → CO2 + 2H2O
(a) (I) and (IV)
(b) (II) only
(c) (I) and (II)
(d) (III) and (IV)
Answer
B
26. Study the table below which shows different colour produced by universal indicator in A, B, C and D
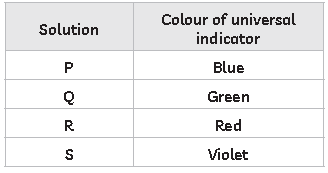
Which of them is strongly basic?
(a) P
(b) Q
(c) R
(d) S
Answer
D
27. An experiment was performed to test the electrical conductivity of some substances as shown in figure below:
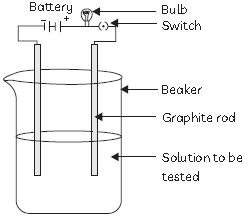
not conduct electricity?
(a) Sodium Chloride
(b) Magnesium Iodide
(c) Alcohol
(d) Calcium oxide
Answer
C
28. Following metals are taken and each of them was tested for its reaction with cold water, hot water and steam: Zn, Al, Cu, Fe, Mg, Na, K Select the correct observations:
(I) Na and K react violently with cold water.
(II) Mg does not react with cold water but reacts with hot water.
(III) Al, Zn and Cu react with steam
(IV) Fe does not react with water or steam at all.
(a) Both (I) and (II)
(b) Both (II) and (III)
(c) Both (I) and (IV)
(d) Both (III) and (IV)
Answer
A
29. When dilute hydrochloric acid is added to iron filling:
(a) Hydrogen gas and iron chloride are formed
(b) Chlorine gas and hydrogen gas evolved
(c) Iron salt and water produced
(d) No reaction takes place
Answer
A
30. If a person is stung by a bee, it causes burning pain and irritation.
Application of which of the following on the stung area can give relief from the pain?
(a) Vinegar
(b) Orange juice
(c) Baking soda
(d) Tomato juice
Answer
C
Question No. 31 to 34 consist of two statements– Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
31. Assertion (A): When hydrogen and chlorine are placed in sunlight, hydrogen chloride is formed
Reason (R): It is an example of combustion reaction.
Answer
C
32. Assertion (A): Most reactive metals react with dilute acids to liberate hydrogen gas.
Reason (R): Very few reactive metals react with bases to liberate hydrogen gas.
Answer
B
33. Assertion (A): The light of violet colour deviates the most and the light of red colour the least, while passing through a prism.
Reason (R): The refractive index is highest for red light and lowest for the violet light.
Answer
B
34. Assertion (A): Focal length of a lens increases when its power decreases.
Reason (R): Power of a lens is inversely proproportional to focal length.
Answer
A
35. Keeping food in air tight containers prevents rancidity of food as it:
(a) Slows down decomposition of foods containing fats and oil.
(b) Slows down oxidation of food containing fats and oil.
(c) Slows down combustion of food containing fats and oil.
(d) Slows down corrosion of foods containing fats and oil.
Answer
B
36. Study the table below and select the row that has the correct information:
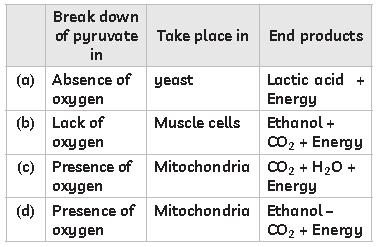
Answer
C
37. The component of blood that helps in clotting of blood is:
(a) Blood plasma
(b) Red Blood Cells
(c) White blood cells
(d) Platelets
Answer
D
38. Observe the diagram of structure of nephron.

Match the labeling referred in column I and correlate with the function or structure in column II.

(a) (I) – (d); (II) –( b); (III) – a); (IV) – (c)
(b) (I) – (a); (II) – (c); (III) – d); (IV) – (b)
(c) (I) – (c); (II) – (d); (III) – b); (IV) – (a)
(d) (I) – (c); (II) – *a); (III) – d); (IV) – (b)
Answer
D
39. What will be the minimum distance between an object and its real image formed by convex lens?
(a) f
(b) >4f
(c) 2f
(d) 4f
Answer
D
40. If the magnification of a lens has a negative value, then the image is:
(a) smaller than object
(b) same height as an object
(c) larger than object
(d) none of these
Answer
A
41. Select the correct statements :
(I) Phloem transports soluble products of photosynthesis in plants.
(II) Xylem transports amino acids and other substances.
(III) Translocation of food and other substances takes place in sieve tubes.
(IV) Translocation in phloem is achieved by utilizing energy.
(a) (I) and (II)
(b) (II) and (III)
(c) (I), (II) and (III)
(d) (I), (III) and (IV)
Answer
D
42. Which of the following event will not take place if the saliva lacks in salivary amylase?
(a) Break down of starch to simple sugar
(b) Breakdown of proteins
(c) Break down of fats
(d) Creating an acidic medium
Answer
A
43. Focal length of a plane mirror is:
(a) – 25
(b) infinite
(c) zero
(d) 25 cm
Answer
B
44. The power of the lens is – 1.0 D. What is the nature of this lens?
(a) Concave lens
(b) Convex lens
(c) Both
(d) Cannot be predicted
Answer
A
45. For a convex mirror, parallel rays of light appear to:
(a) diverge
(b) converge
(c) remains parallel
(d) none of the above
Answer
A
46. A student tabulated the following information based on his studies of optical phenomena.
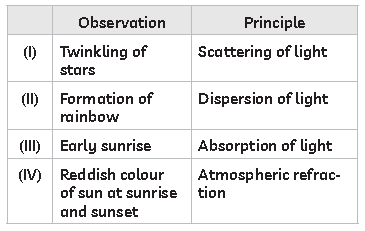
Select the row/s which contain correct observation:
(a) Only (I)
(b) Only (II)
(c) (I) and (III)
(d) (II) and (IV)
Answer
B
47. Rajendra conducted an experiment using a concave mirror with a focal length of 30 cm.
He places an object at a distance of 20 cm in front of the mirror. The image is formed
(a) 20 cm in front of the mirror
(b) 10 cm in front of the mirror
(c) 30 cm behind the mirror
(d) 60 cm behind the mirror
Answer
D
48. A compound Z is manufactured by the action of a gas X, which is a product of Chlor alkali process, on a substance Y in a plant as shown:
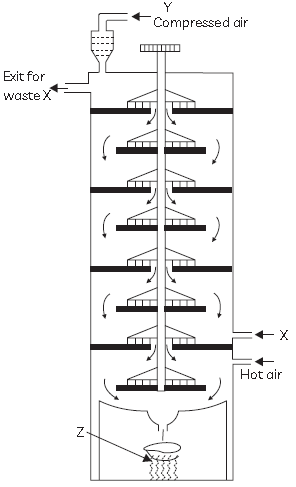
Z is used as an oxidising agent in many chemical industries and also used for disinfecting drinking water. Select the row containing the correct identification of X, Y and Z from the table below:
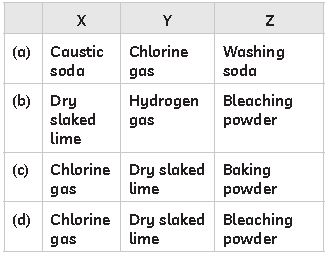
Answer
D
SECTION-C
Q. 49-52 are based on Case Study–1.
Case 1: When an element composed of atoms that readily lose electrons (a metal) reacts with an element composed of atoms that readily gain electrons (a nonmetal), a transfer of electrons usually occurs, producing ions. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound.
For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, Na+, and each chlorine atom in a sample of chlorine gas (group 17) accepts one electron to form a chloride anion, Cl−, the resulting compound, NaCl, is composed of sodium ions and chloride ions in the ratio of one Na+ ion for each Cl− ion.

Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form CaCl2, which is composed of Ca2+ and Cl− ions in the ratio of one Ca2+ ion to two Cl− ions. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are ionic: Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. The melting and boiling points of some common compounds is given below.
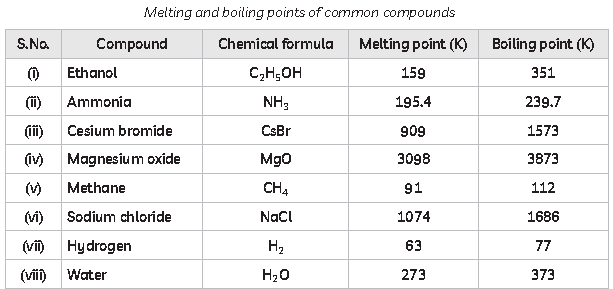
49. Which of the compounds given in table above are ionic compounds?
(I) Magnesium oxide
(II) Sodium chloride
(III) Ammonia
(IV) Cesium bromide
(a) Both (I) and (II)
(b) (I), (II) and (III)
(c) (I), (II) and (IV)
(d) Both (IV) and (III)
Answer
C
50. The atomic number of four elements P, Q, R, S are 10, 12, 14 and 16 respectively. The two elements which can react to form ionic compounds are:
(a) P and S
(b) Q and R
(c) P and R
(d) Q and S
Answer
D
51. Which of the following statement is true about ionic compounds?
(I) Ionic compounds are crystalline solids.
(II) Ionic compounds are soluble in solvents such as kerosene and petrol.
(III) Ionic compounds conduct electricity when dissolved in water.
(IV) Ionic compounds conduct electricity in the molten state.
(a) Both (I) and (II)
(b) Both I and (III)
(c) (I), (III) and (IV)
(d) (I), (II) and (IV)
Answer
C
52. An element X having atomic number 13 forms a compound with element Y having atomic number 9. The cations and anions formed will be:
(a) 3[X+] and [Y3–]
(b) [X3+] and 3[Y–]
(c) 3[X+] and 3[Y–]
(d) [X3+] and [Y3-]
Answer
B
Q. 53-56 are based on Case Study–2.
Case 2: Haemoglobin (Hb) is a protein found in the red blood cells that carries oxygen in your body and gives blood its red colour. Haemoglobin levels vary from person to person. Men usually have higher levels than women. A haemoglobin “cutoff” level is set for blood donation to ensure that your haemoglobin will not drop below normal after you have donated blood. Normal ranges for haemoglobin differ between ethnic populations, males and females, and are also affected by age, especially in women. The haemoglobin level is expressed as the amount of haemoglobin in grams (g) per deciliter (dL) of whole blood, a deciliter being 100 milliliters.
53. The graph depicts a patient’s haemoglobin levels over a 45 days period. Study the graph and answer the question below:
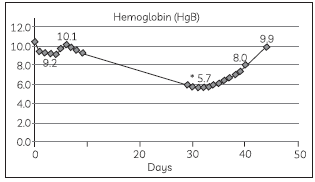
The condition the patient is suffering from caused due to a low level of haemoglobin is:
(a) Asthma
(b) Emphysema
(c) Respiratory alkalosis
(d) Anemia
Answer
D
54. The table below lists some parts of the human respiratory system. Select the row containing incorrect information.
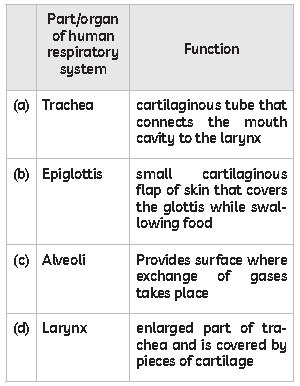
Answer
A
55. The normal ranges for Haemoglobin are:
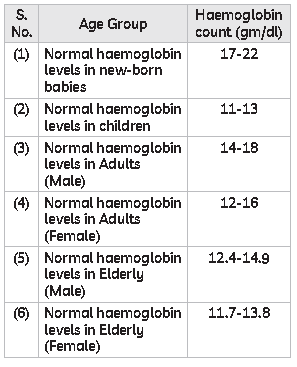
Which of the following helps our body to make haemoglobin?
(a) Iron
(b) Sodium
(c) Calcium
(d) Both (a) and (b)
Answer
A
56. Study the graph below that illustrates the difference between the content in blood of oxygen and carbon dioxide with change in partial pressure (partial pressure is the pressure of that constituent gas if it alone occupied the entire volume of the original mixture at the same temperature. Gases dissolve, diffuse, and react according to their partial pressures).
Now answer the question that follows:
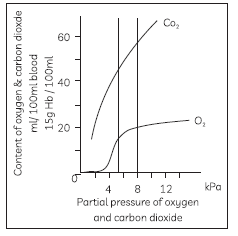
Carbon dioxide is transported in our body:
(a) By haeomoglobin
(b) In dissolved form
(c) By ATP
(d) By alveoli
Answer
B
Q. 57-60 are based on Case Study–3.
Case 3: Dispersion of light occurs when white light is separated into its different constituent colors because of refraction and Snell’s law. From Snell’s law it can be seen that the angle of refraction of light in a prism depends on the refractive index of the prism material.

Since the refractive index varies with wavelength, the angle that the light is refracted by will also vary with wavelength, causing an angular separation of the colors known as angular dispersion.
For visible light, refraction indices n of most transparent materials (e.g., air, glasses) decrease with increasing wavelength :

Most often seen in recently made puddles on the sides of roads, the oil refracts light much the same way a rainbow does. Simply put, the thin layer of oil floating on top of the water refracts the light which then bounces back up off the water underneath, splitting the light rays creating a pool of rainbow colours.
57. The ray which is least deviated by a prism is:
(a) Violet ray
(b) Green ray
(c) Red ray
(d) Yellow ray
Answer
C
58. The colour of light which has the minimum velocity in the glass prism is:
(a) Red
(b) Green
(c) Blue
(d) Violet
Answer
D
59. The angle of deviation (D) of a prism is :
(a) The angle between incident and refracted ray
(b) The angle between the emergent and refracted ray
(c) The angle between the incident and emergent ray
(d) The angle between emergent ray and prism surface
Answer
C
60. A student plotted the following graph to observe how the refractive index of the material of a prism varies with the wavelength of different components of light.
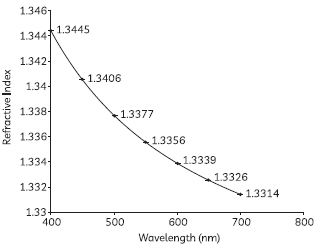
Answer
C

