Please refer to Hydrocarbons MCQ Questions Class 11 Chemistry below. These MCQ questions for Class 11 Chemistry with answers have been designed as per the latest NCERT, CBSE books, and syllabus issued for the current academic year. These objective questions for Hydrocarbons will help you to prepare for the exams and get more marks.
Hydrocarbons MCQ Questions Class 11 Chemistry
Please see solved MCQ Questions for Hydrocarbons in Class 11 Chemistry. All questions and answers have been prepared by expert faculty of standard 11 based on the latest examination guidelines.
MCQ Questions Class 11 Chemistry Hydrocarbons
Question. Ethylene can be converted into alcohol by treatment with
(a) aq KOH
(b) dil. H2SO4
(c) moist silver oxide
(d) Zn/HCI
Answer
B
Question. Which of the following is the predominant product in the reaction of HO Br with propene?
(a) 2-bromo-1-propanol
(b) 3-bromo-1-propanol
(c) 2-bromo-2-propanol
(d) l-bromo-2-propanol
Answer
D
Question. In the series,
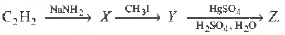
The compound Z is
(a) CH3CH2CH = CH2
(b) CH3COCH3
(c) CH3CHO
(d) CH3CH2CH2CHO
Answer
B
Question. Propyne on passing through red hot iron tube gives

Answer
A
Question. Ozonolysis products of an olefin are
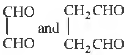

Answer
C
Question. CH3 — CH = CH2 + NOCI → P
Identify the adduct

Answer
A
Question. Identify Bin the following reaction,
CH2=CH2 + HCl →Anhy.AICl3 A + 2[H] →Zn-Cu B + HCI
C2H5OH
(a) CH4
(b) C2H6
(c) C2H5CI
(d) C2H5OH
Answer
B
Question. Which one of the following has the minimum boiling point?
(a) n-butane
(b) 1-butyne
(c) I-butene
(d) Iso-butene
Answer
D
Question. The compound with highest boiling point
(a) n-hexane
(b) n-pentane
(c) 2, 2-dimethylpropane
(d) 2-methylbutane
Answer
A
Question. When acetylene is passed through dil. H2SO4 in presence of HgSO4 , the compound fo1med is
(a) ether
(b) acetaldehyde
(c) acetic acid
(d) ketone
Answer
B
Question. Propyne on ozonolysis gives
(a) CH3COOH + HCOOH
(b) CH3COOH + HCHO
(c) CH3CHO + HCHO
(d) CH3CHO + HCOOH
Answer
A
Question. Identify Band Din the following sequence ofreactions.
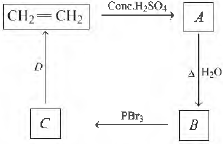
(a) Methanol and bromoethane
(b) Ethyl hydrogen sulphate and alcoholic KOH
(c) Ethyl hydrogen sulphate and aqueous KOH
(d) Ethanol and alcoholic KOH
Answer
D
Question. 2-hexyne gives trans-2-hexene on treatment with
(a) Pt/H2
(b) Li/NH3
(c) Pd/BaSO4
(d) LiAlH4
Answer
B
Question. One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44 u. The alkene is
(a) propene
(b) I-butene
(c) 2-butene
(d) ethene
Answer
C
Question. Which compound does not give precipitate with anlflloniacal silver nitrate solution?
(a) C2H5—C= CH
(b) CH3—C=C—CH3
CH3
l
(c) CH3—CH—C=CH
(d) Ph—CH2—C=CH
Answer
B
Question. The nwnber of optically active products obtained from the complete ozonolysis of the given compound

(a) 0
(b) 1
(c) 2
(d) 4
Answer
A
Question. Which of the following fraction of coaltar distillation is obtained at 270°-360°C?
(a) Light oil
(b) Middle oil
(c) Green oil
(d) Heavy oil
Answer
C
Question. Cetane is a compound which has very good ignition property. Chemically it is
(a) CH3 (CH2 )14 CH3
(b) (CH3)3 C(CH2 )11 CH3
(c) C17H34
(d) None of the above
Answer
A
Question. LPG mainly contains
(a) ethyne
(b) butane
(c) methane
(d) ethane
Answer
B
Question. Petroleum is obtained from water gas, name of the reaction involved is
(a) Fischer-Tropsch
(b) Bengoic
(c) Dow’s
(d) Kjeldahl’s
Answer
A
Question. Which of the following is correct number of carbon atom present as the constituent of kerosene oil ?
(a) C10—C16
(b) C4—C6
(c) C8—C16
(d) C12—C18
Answer
A
Question. Which of the following increases the octane number ?
(a) Branching of chain
(b) Absence of double and triple bond
(c) Non-cyclic alkanes
(d) None of the above
Answer
A
Question. Tetraethyl lead is a
(a) solvent
(b) petroleum additive
(c) oxidising agent
(d) fire extinguisher
Answer
B
Question. In laboratory burners, we use
(a) producer gas
(b) oil gas
(c) gobar gas
(d) coal gas
Answer
B
Question. Which of the following is present in natw-al gas ?
(a) n-butane
(b) Ethane
(c) Methane
(d) Propane
Answer
C
Question. Isomers of hexane, based on their branching can be divided into three distinct classes as shown in the figure. The correct order of their boiling point is
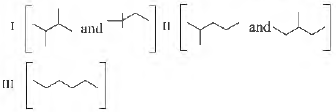
(a) I > II > III
(b) III > II > I
(c) II > III > I
(d) III > I > II
Answer
B
Question. Which of the following alkenes gives an acetaldehyde on ozonolysis ?
(a) Ethene
(b) Propene
(c) 1 -butene
(d) 2-butene
Answer
D
Question. When C2H2, CH4 and C2H4 passes through a test tube which have ammoniacal Cu2Cl2, find out which gas comes out unaffected from test tube ?
(a) C2H2 and CH4
(b) C2H2 and C2H4
(c) C2H2 and CH4
(d) C2H2
Answer
C
Question. In the following reaction,
C2H2 →H2O X ⇌ CH3CHO.
HgSO4/H2SO4
What is X ?
(a) CH3CH2OH
(b) CH3 — O — CH3
(c) CH3CH2CHO
(d) CH2 = CHOH
Answer
D
Question. Acetylene and HCHO react in presence of copper acetylide catalyst to form
(a) 1-butyne-1, 4-diol
(b) 2-butyne-1, 2-diol
(c) 2-butyne-1 , 4-diol
(d) None of these
Answer
C
Question. In the reactions,
Lindlar catalyst/ H2 Na/NH
B ← RC = CR → A
H2
A and B are geometrical isomers. Then,
(a) A is cis and Bis trans
(b) A is trans and B is cis
(c) A and Bare cis
(d) A and B are transA
Answer
B
Question. Which branched chain isomer of the hydrocarbon with molecular mass 72u gives only one isomer of mono substituted alkyl halide?
(a) Te1tiarybutyl chloride
(b) Neo-pentane
(c) ]so-hexane
(d) Neo-hexane
Answer
B
Question. The treatment of CH3MgX with CH3C=C- H produces
(a) CH3 -CH= CH2
(b) CH3C = C—CH3
H H
l l
(c) CH3 —C= C—CH3
(d) CH4
Answer
D
Question. What are X and Y respectively in the following reaction?
Z-product ←Y 2-butyne →X E- product
(a) Na/NH3 (liq.) and Pd/BaSO4 + H2
(b) Ni/140°C and Pd/BaSO4 + H2
(c) Ni/140°C and Na/NH3(liq.)
(d) Pd/BaSO4 + H2 and Na/NH3(liq.)
Answer
A
Question. An alkene on vigorous oxidation with KMnO4 gives only acetic acid. The alkene is
(a) CH3CH2CH = CH2
(b) CH3CH = CHCH3
(c) (CH3)2C=CH2
(d) CH3CH= CH2
Answer
B
Question. Which one of the following compounds will react with methyl magnesium bromide?
(a) CH3CH2CH2CH2CH3
(b) CH3CH = CH—CH= CH2
(c) CH3 —C = C—CH2CH3
(d) CH3CH2CH2C = CH
Answer
D
Question. Which of the following is correct order according to boiling point?
(a) n-pentane < n-hexane < 2, 3-dimethyl butane
(b) n-pentane < 2, 3-dirnethyl butane< n-hexane
(c) 2, 3-din1ethyl butane< n-hexane < n-pentane
(d) n-hexane < 2, 3-dirnethyl butane< n-pentane
Answer
B
Question. The major organic compound formed by the reaction of 1, 1, 1- trichloroethane with silver powder is
(a) acetylene
(b) ethene
(c) 2-butyne
(d) 2-butene
Answer
C
Question. Which of the following is correct order according to boiling point?
(a) n-pentane < n-hexane < 2, 3-dimethyl butane
(b) n-pentane < 2, 3-dirnethyl butane< n-hexane
(c) 2, 3-din1ethyl butane< n-hexane < n-pentane
(d) n-hexane < 2, 3-dirnethyl butane< n-pentane
Answer
B
Question. Ammoniacal cuprous chloride will give red precipitate with which one of the following ?
(a) CH3 —C = C—CH3
(b) CH3 —CH = CH2
(c) CH3 —C=CH
(d) CH3 —CH= CH—CH3
Answer
C
Question. The dihalogen derivative X of a hydrocarbon with three carbon atoms reacts with alcoholic KOH and produces another hydrocarbon which forms a red precipitate with ammoniacal Cu2Cl2 . X gives an aldehyde on reaction with aqueous KOH. The compound Xis
(a) 1, 3-dichloropropane
(b) 1, 2-dichloropropane
(c) 2, 2-dichloropropane
(d) 1, 1-dichloropropane
(e) 1, 3-dichloropropene
Answer
A
Question. Which one of the following gives, on ozonolysis, both aldehydes and ketones ?
(a) Me2C==CHMe
(b) Me2C=CMe2
(c) MeCH2— C(Me) = CMe2
(d) MeCH(Me)—CH = CHMe
Answer
B
Question. The Markownikoff’s rule is the best applicable to the reaction between
(a) C2H4 + HCI
(b) C3H6 + Br2
(c) C3H6 + HBr
(d) C3H8 + Cl2
(e) C2H4 + I2
Answer
C
Question. Propene on reaction with hypochlorous acid to give

Answer
A
Question. An alkene on reductive ozonolysis gives 2-molecules of CH2 (CHO)2 . The alkene is
(a) 2, 4-hexadiene
(b) 1, 3-cyclohexadiene
(c) 1, 4-cyclohexadiene
(d) 1-methyl- 1, 3-cyclopentadiene
(e) 1, 2-dirnethyl cyclopropene
Answer
C
Question. HBr reacts with CH2= CH—OCH3 under anhydrous conditions at room temperature to give
(a) CH3CHO and CH3Br
(b) BrCH2CHO and CH3OH
(c) BrCH2 — CH2 — OCH3
(d) H3C — CHBr— OCH3
Answer
D
Question. Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound Eon further treatment with aqueous KOH yields compound F. Compound F is
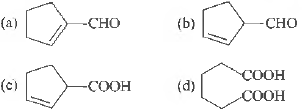
Answer
A
Question. In the reaction sequence, Products will be
CH3CH=CH2 →(i)O3 Products will be
(ii) H2O/Zn
(a) CH3 COCH3
(b) CH3 COCH2OH
(c) CH3 COOH + HCOOH
(d) CH3 CHO + HCHO
Answer
D
Question. 2-methylbutane on reacting with bromine in the presence of sunlight gives mainly
(a) l-bromo-3-methylbutane
(b) 2-bromo-3-methylbutane
(c) 2-bromo-2-methylbutane
(d) l-bromo-2-methylbutane
Answer
C
Question. When a mixture of methane and oxygen is passed through heated molybdenum oxide, the main product formed is
(a) methanoic acid
(b) ethanal
(c) methanol
(d) methanal
Answer
D
Question. Ethylene reacts with 1 % alkaline KMnO4 to form
(a) oxalic acid
(b) ethylene glycol
(c) ethyl alcohol
(d) HCHO
Answer
B
Question. What is the product formed when acetylene reacts with hypochlorous acid ?
(a) CH3COCI
(b) ClCH2CHO
(c) Cl2CHCHO
(d) ClCH2COOH
Answer
C
Question. A compoundX on ozonolysis followed by reduction gives an aldehyde C2H4O and 2-butanone. Compound Xis
(a) 3-methyl pentene-2
(b) 3-methyl pentene-3
(c) 3-methyl hexene-3
(d) 3-ethyl pentene-3
Answer
A
Question. Select the reagent for the following reaction,

(a) SeO2
(b) O3 ,Zn/ H2O
(c) O3, H2O2-CH3COOH
(d) PCC
Answer
B
Question. According to Markownikoffs rule, what will be the major product of reaction,
CH2= CH—CH3 →HBr ?
Br
l
(a) CH3—CH—CH3
(b) Br—CH2—CH2—CH3
(c) CH2= CH—CH2Br
(d) CH2=C=CH2
Answer
A
Question. Propyne on passing through red hot copper tube forms
(a) benzene
(b) toluene
(c) mesitylene
(d) None of these
Answer
C
Question. Mustard gas is a
(a) oil gas
(b) poisonous gas
(c) fuel gas
(d) life gas
Answer
B
Question. In the following reaction,
RCH2CH= CH2 + ICI → A
Markownikoff’s product A is
(a) RCH2 CH—CH2I
l
Cl
(b) RCH2 CH—CH2CI
l
I
(c) RCH2—C=CH2
l
I
(d) RCH=CH—CH2I
Answer
A
Question. CH3 CH3 + HNO3 →675 K ?
(a) CH3CH2NO2
(b) CH3CH2NO2 + CH3NO2
(c) 2CH3NO2
(d) CH2 = CH2
Answer
B



