Please refer to Thermodynamics MCQ Questions Class 11 Physics below. These MCQ questions for Class 11 Physics with answers have been designed as per the latest NCERT, CBSE books, and syllabus issued for the current academic year. These objective questions for Thermodynamics will help you to prepare for the exams and get more marks.
Thermodynamics MCQ Questions Class 11 Physics
Please see solved MCQ Questions for Thermodynamics in Class 11 Physics. All questions and answers have been prepared by expert faculty of standard 11 based on the latest examination guidelines.
MCQ Questions Class 11 Physics Thermodynamics
Question. Which of the following is incorrect regarding first law of thermodynamics?
(a) It is a restatement of principle of conservation of energy.
(b) It is applicable to cyclic processes
(c) It introduces the concept of entropy
(d) It introduces the concept of internal energy
Answer
C
Question. Choose the incorrect statement related to an isobaric process.
(a) V/T = constant
(b) W = PΔV
(c) Heat given to a system is used up in raising the temperature only.
(d) ΔQ > W
Answer
C
Question. The internal energy of an ideal gas does not depend upon
(a) temperature of the gas
(b) pressure of the gas
(c) atomicity of the gas
(d) number of moles of the gas.
Answer
B
Question. Air conditioner is based on the principle of
(a) Carnot cycle
(b) refrigerator
(c) first low of thermodynamics
(d) None of these
Answer
B
Question. During isothermal expansion, the slope of P-V graph
(a) decreases
(b) increases
(c) remains same
(d) may increase or decrease
Answer
A
Question. During melting of ice, its entropy
(a) increases
(b) decreases
(c) remains same
(d) cannot say
Answer
A
Question. A mass of ideal gas at pressure P is expanded isothermally to four times the original volume and then slowly compressed adiabatically to its original volume. Assuming g to be 1.5, the new pressure of the gas is
(a) 2 P
(b) P
(c) 4 P
(d) P/2
Answer
A
Question. The efficiency of carnot engine when source temperature is T1 and sink temperature is T2 will be

Answer
A
Question. One mole of an ideal gas at temperature T was cooled isochorically till the gas pressure fell from P to P/n . Then, by an isobaric process, the gas was restored to the initial temperature. The net amount of heat absorbed by the gas in the process is
(a) nRT
(b) RT/n
(c) RT (1 – n–1)
(d) RT (n – 1)
Answer
C
Question. For an ideal gas graph is shown for three processes. Process 1, 2 and 3 are respectively.
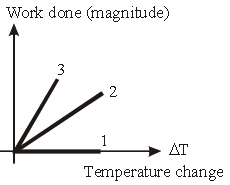
(a) Isobaric, adiabatic, isochoric
(b) Adiabatic, isobaric, isochoric
(c) Isochoric, adiabatic, isobaric
(d) Isochoric, isobaric, adiabatic
Answer
D
Question. In the equation PVy = constant, the value of g is unity. Then the process is
(a) isothermal
(b) adiabatic
(c) isobaric
(d) irreversible
Answer
A
Question. In changing the state of thermodynamics from A to B state, the heat required is Q and the work done by the system is W. The change in its internal energy is
(a) Q + W
(b) Q – W
(c) Q
(d) Q-W/2
Answer
B
Question. For adiabatic processes (Letters have usual meanings)
(a) PyV = constant
(b) TyV = constant
(c) TVy –1 = constant
(d) TVy = constant
Answer
C
Question. Volume of one mole gas changes according to the V = a/T. If temperature change is ΔT, then work done will be
(a) RΔT
(b) – RΔT
(c) (R/y – 1) ΔT
(d) R (g – 1) ΔT
Answer
B
Question. If ΔQ and ΔW represent the heat supplied to the system and the work done on the system respectively, then the first law of thermodynamics can be written as
(a) ΔQ = ΔU + ΔW
(b) ΔQ = ΔU – ΔW
(c) ΔQ = ΔW – ΔU
(d) ΔQ = –ΔW – ΔU
Answer
B
Question. A gas at 27ºC and pressure of 30 atm. is allowed to expand to atmospheric pressure and volume 15 times larger. The final temperature of the gas is
(a) – 123ºC
(b) +123ºC
(c) 273ºC
(d) 373ºC
Answer
A
Question. The work done in which of the following processes is equal to the internal energy of the system?
(a) Adiabatic process
(b) Isothermal process
(c) Isochoric process
(d) None of these
Answer
A
Question. Which of the following processes is reversible?
(a) Transfer of heat by conduction
(b) Transfer of heat by radiation
(c) Isothermal compression
(d) Electrical heating of a nichrome wire
Answer
C
Question. A system changes from the state (P1, V1) to (P2, V2) as shown in the figure. What is the work done by the system?
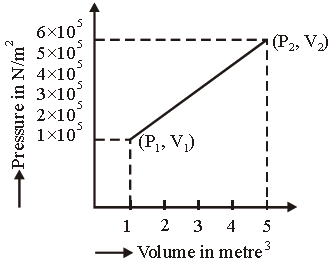
(a) 7.5 × 105 joule
(b) 7.5 × 105 erg
(c) 12 × 105 joule
(d) 6 × 105 joule
Answer
C
Question. The temperature of 5 moles of a gas which was held at constant volume was changed from 100º to 120ºC. The change in the internal energy of the gas was found to be 80 joule, the total heat capacity of the gas at constant volume will be equal to
(a) 8 joule per K
(b) 0.8 joule per K
(c) 4.0 joule per K
(d) 0.4 joule per K
Answer
C
Question. A polyatomic gas (g = 4/3) is compressed to 1/8th of its volume adiabatically. If its initial pressure is P0, its new pressure will be
(a) 8P0
(b) 16P0
(c) 6P0
(d) 2P0
Answer
B
Question. Three copper blocks of masses M1, M2 and M3 kg respectively are brought into thermal contact till they reach equilibrium. Before contact, they were at T1, T2, T3 (T1 > T2 > T3). Assuming there is no heat loss to the surroundings, the equilibrium temperature T is (s is specific heat of copper)

Answer
B
Question. A gas is taken through the cycle A → B → C → A, as shown in figure. What is the net work done by the gas ?

(a) 1000 J
(b) zero
(c) – 2000 J
(d) 2000 J
Answer
A
Question. The efficiency of a Carnot engine operating with reservoir temperatures of 100ºC and –23ºC will be

Answer
C
Question. At 27ºC a gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (g = 5/3)
(a) 450 K
(b) 300 K
(c) –142ºC
(d) 327ºC
Answer
C
Question. A diatomic gas initally at 18ºC is compressed adiabatically to one eighth of its original volume. The temperature after compression will be
(a) 18ºC
(b) 887ºC
(c) 327ºC
(d) None of these
Answer
D
Question. Absolute zero is obtained from
(a) P–V graph
(b) P – ( 1/V)graph
(c) P–T graph
(d) V–T graph
Answer
C
Question. An ideal gas heat engine operates in Carnot cycle between 227°C and 127°C. It absorbs 6 × 104 cals of heat at higher temperature. Amount of heat converted to work is
(a) 4.8 × 104 cal
(b) 6 × 104 cal
(c) 2.4 × 104 cal
(d) 1.2 × 104 cal
Answer
D
Question. Three moles of an ideal gas kept at a constant temperature at 300 K are compressed from a volume of 4 litre to 1 litre. The work done in the process is
(a) – 10368 J
(b) –110368 J
(c) 12000 J
(d) 120368 J
Answer
A
Question. An ideal refrigerator has a freezer at a temperature of 13ºC. The coefficient of performance of the engine is 5. The temperature of the air (to which heat is rejected) is
(a) 320ºC
(b) 39ºC
(c) 325 K
(d) 325ºC
Answer
B
Question. One mole of an ideal monoatomic gas is heated at a constant pressure of one atmosphere from 0ºC to 100ºC. Then the work done by the gas is
(a) 6.56 joule
(b) 8.32 × 102 joule
(c) 12.48 × 102 joule
(d) 20.8 × 102 joule
Answer
B
Question. The pressure inside a tyre is 4 times that of atmosphere. If the tyre bursts suddenly at temperature 300 K, what will be the new temperature?
(a) 300 (4)7/2
(b) 300 (4)2/7
(c) 300 (2)7/2
(d) 300 (4)–2/7
Answer
D
Question. A monatomic ideal gas expands at constant pressure, with heat Q supplied. The fraction of Q which goes as work done by the gas is
(a) 1
(b) 2/3
(c) 3/5
(d) 2/5
Answer
D
Question. A carnot’s engine takes 300 calories of heat at 500 K and rejects 150 calories of heat to the sink. The temperature of the sink is
(a) 1000 K
(b) 750 K
(c) 250 K
(d) 125 K
Answer
C
Question. The source and sink temperatures of a Carnot engine are 400 K and 300 K, respectively. What is its efficiency?
(a) 100%
(b) 75%
(c) 33.3%
(d) 25%
Answer
D
Question. The volume of a gas is reduced adiabatically to 1/4 of its volume at 27ºC. If g = 1.4 the new temperature is
(a) (300) 20.4 K
(b) (300) 21.4 K
(c) 300 (4)0.4 K
(d) 300 (2)1.4 K
Answer
C
Question. In pressure-volume diagram, the isochoric, isothermal, isobaric and iso-entropic parts respectively, are
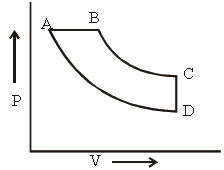
(a) BA, AD, DC,CB
(b) DC, CB, BA, AD
(c) AB, BC, CD, DA
(d) CD, DA, AB, BC
Answer
D
Question. A Carnot’s engine works as a refrigerator between 250 K and 300 K. If it receives 750 calories of heat from the reservoir at the lower temperature, the amount of heat rejected at the higher temperature is
(a) 900 calories
(b) 625 calories
(c) 750 calories
(d) 1000 calories
Answer
A
Question. A Carnot engine is working between 127°C and 27°C. The increase in efficiency will be maximum when the temperature of
(a) the source is increased by 50°C
(b) the sink is decreased by 50°C
(c) source is increased by 25°C and that of sink is decreased by 25°C
(d) both source and sink are decreased by 25°C each.
Answer
B
Question. In an adiabatic process, the pressure is increased by (2/3) % If g = 3/2 , then the volume decreases by nearly

Answer
A
Question. A closed gas cylinder is divided into two parts by a piston held tight. The pressure and volume of gas in two parts respectively are (P, 5V) and (10P, V). If now the piston is left free and the system undergoes isothermal process, then the volumes of the gas in two parts respectively are
(a) 2V, 4V
(b) 3V, 3V
(c) 5V, V
(d) (10/11)V, (20/11)V
Answer
A
Question. Figure shows the variation of internal energy (U) with the pressure (P) of 2.0 mole gas in cyclic process abcda. The temperature of gas at c and d are 300 K and 500 K. Calculate the heat absorbed by the gas during the process.
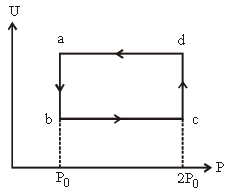
(a) 400 R ln 2
(b) 200 R ln 2
(c) 100 R ln 2
(d) 300 R ln 2
Answer
A
Question. The figure shows the P-V plot of an ideal gas taken through a cycle ABCDA. The part ABC is a semi-circle and CDA is half of an ellipse. Then,
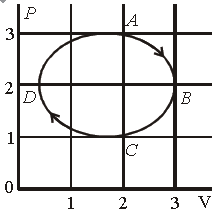
(a) the process during the path A → B is isothermal
(b) heat flows out of the gas during the path B → C → D
(c) work done during the path A → B → C is zero
(d) positive work is done by the gas in the cycle ABCDA
Answer
B, D
Question. One mole of an ideal gas at an initial temperature of T K does 6R joules of work adiabatically. If the ratio of specific heats of this gas at constant pressure and at constant volume is 5/3, the final temperature of gas will be
(a) (T – 4) K
(b) (T + 2.4) K
(c) (T – 2.4) K
(d) (T + 4) K
Answer
A
Question. If ΔU and ΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true?
(a) ΔU = -ΔW, in an adiabatic process
(b) ΔU = ΔW, in an isothermal process
(c) ΔU = ΔW, in an adiabatic process
(d) ΔU =-ΔW, in an isothermal process
Answer
A
Question. During an isothermal expansion, a confined ideal gas does –150 J of work against its surroundings. This implies that
(a) 150 J heat has been removed from the gas
(b) 300 J of heat has been added to the gas
(c) no heat is transferred because the process is isothermal
(d) 150 J of heat has been added to the gas
Answer
A, D
Question. A thermodynamic system is taken through the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is
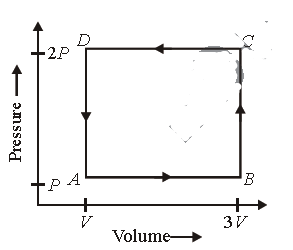
(a) 2 PV
(b) 4 PV
(c) (1/2) PV
(d) P V
Answer
A
Question. An ideal gas goes from state A to state B via three different processes as indicated in the P-V diagram :
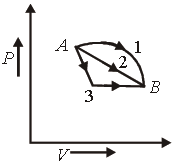
If Q1, Q2, Q3 indicate the heat a absorbed by the gas along the three processes and ΔU1, ΔU2, ΔU3 indicate the change in internal energy along the three processes respectively, then
(a) Q1 > Q2 > Q3 and ΔU1 = ΔU2 = ΔU3
(b) Q3 > Q2 > Q1 and ΔU1= ΔU2 = ΔU3
(c) Q1 = Q2 = Q3 and ΔU1 > ΔU2 > ΔU3
(d) Q3 > Q2 > Q1 and ΔU1> ΔU2 > ΔU3
Answer
A
Question. In the given (V – T) diagram, what is the relation between pressure P1 and P2 ?
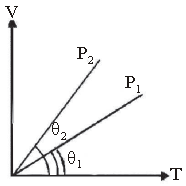
(a) P2 > P1
(b) P2 < P1
(c) P2 = P1
(d) Cannot be predicted
Answer
B
Question. A system goes from A to B via two processes I and II as shown in figure. If ΔU1 and ΔU2 are the changes in internal energies in the processes I and II respectively, then
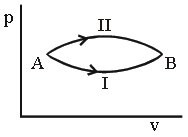
(a) relation between ΔU1 and ΔU2 can not be determined
(b) ΔU1 = ΔU2
(c) ΔU1 < ΔU2
(d) ΔU1 > ΔU2
Answer
B

