Please refer to the Chemical Reactions and Equations Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 10th Science book. We have provided chapter wise Notes for Class 10 Science as per the latest examination pattern.
Revision Notes Chapter 1 Chemical Reactions and Equations
Students of Class 10 Science will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 10 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
Chemical Changes and Their Representation in the Form of Chemical Equations
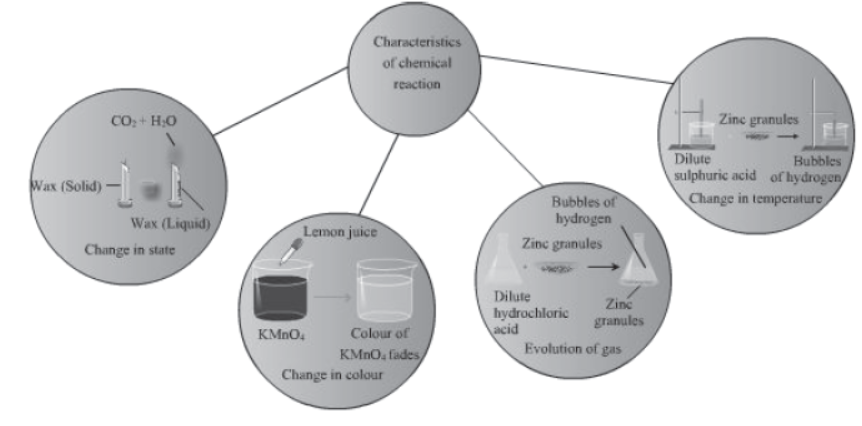
A chemical change can be confirmed by any or all of the following observations-
- change in state
- change in colour
- change in temperature
- evolution of gas
- formation of a precipitate
A chemical change is always accompanied by a chemical reaction. Reaction is the term used for depicting a change or transformation in which a substance decomposes, combines with other substances, or interchanges constituents with other substances.
Thus, a chemical equation is an easier and more concise method for representing a chemical reaction. It involves writing symbols and formulae (instead of words) for all substances involved in the reaction. A chemical equation also indicates the number of atoms of each element involved in a reaction. In which reactants are given on left-hand side of a reaction and products are given on right-hand side.
Reactants: The substance which takes part in a chemical reaction.
Products: The new substances produced as a result of chemical reaction.
Try to represent the statements given below as chemical equations.
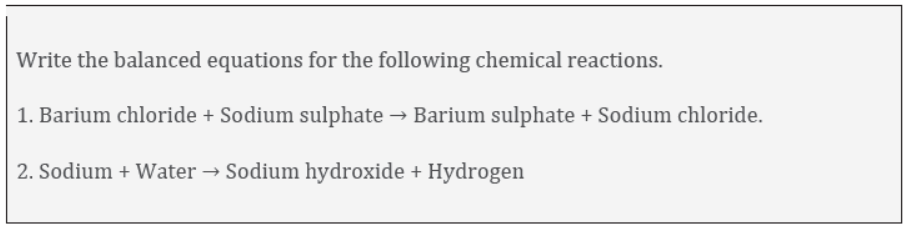
(a) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
(b) Hydrogen gas combines with nitrogen to form ammonia.
Symbols of elements:
Potassium = K
Hydrogen =H
Nitrogen = N
Ques. Write any two observations in an activity which may suggest that a chemical reaction has taken place. Give an example in support of your answer.
Sol: A chemical change can be confirmed by any of the following observations:
- Change in temperature
- Evolution of gas
For example: Calcium oxide reacts vigorously with water to produce calcium hydroxide.
During this process, a large amount of heat is also evolved, which increases the temperature of the system. This confirms that a chemical reaction has taken place.
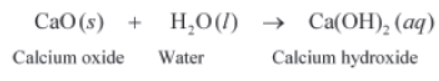
Also, when calcium carbonate is heated, it decomposes to form calcium oxide and carbon dioxide.

In this reaction, calcium carbonate breaks down to form calcium oxide and carbon dioxide.
Here, evolution of the gas (carbon dioxide) confirms that a chemical reaction has taken place.
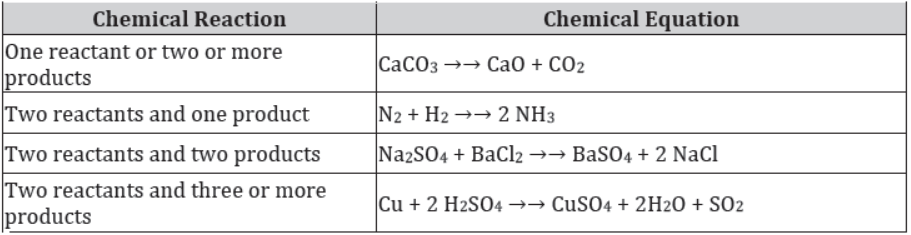
Balanced Chemical Equations
To describe a chemical reaction more concisely, equations of the reactions are written.
Chemical equation
A chemical equation is a concise form which uses symbols and formulae of the chemical compounds or elements involved in the reaction. It also indicates the number of atoms of each element involved in a reaction.
In a chemical reaction, the total mass of the reactants should be equal to the total mass of the products. This means that the total number of atoms of each element should be equal on both sides of a chemical equation. Such an equation is called a balanced chemical equation, and the method by which it is obtained is called the balancing of chemical equations.
Another example of a balanced chemical equation is the reaction of limewater with carbon dioxide, that results in the formation of a precipitate of calcium carbonate and water is represented as:

In this reaction, calcium hydroxide is present in the form of a solution in water, carbon dioxide is present as a gas, calcium carbonate is produced as a precipitate i.e. in the solid state, and water is formed in the liquid state.
The energy changes involved in a reaction are denoted by writing the changes involved in the equation itself.
If energy is used in the reaction, then it will be written on the left-hand side. If it is released in the process, then it is written on the right-hand side.
For example, combustion of butane (or any other hydrocarbon i.e., the compounds made up of carbon and hydrogen) is accompanied by the evolution of heat and light energy along with the production of carbon dioxide and water. Therefore, the equation for the same will be written as:
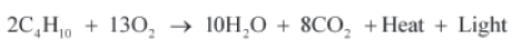
The reaction conditions (such as temperature, pressure, catalyst etc.) for a reaction are indicated above or below the forward arrow in a reaction.
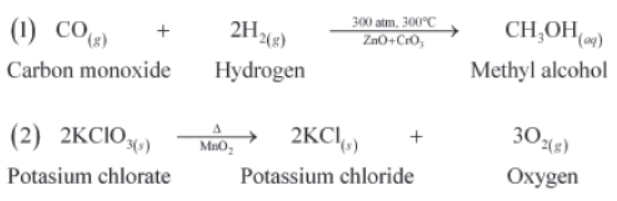
Below are some balanced chemical equations:
Ques. On what basis is a chemical equation balanced?
Sol: Law of conservation of mass forms the basis of balancing chemical equations. In a balanced chemical equation, the number of atoms of each element is equal on both sides of the equation.
Ques. Balance the following chemical equation:
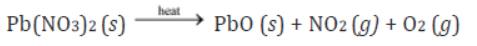
Sol: The balanced chemical equation for the given reaction is:
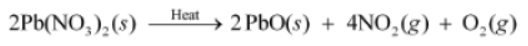
Information conveyed by balanced chemical equations
- Result of the chemical change
- Number of molecules of reactants being consumed and products being formed
- Chemical composition of reactants and product species
- Molecular mass of reactants and products
- Proves the law of conservation of mass
Limitation of a chemical equation
A chemical equation does not provide some other important chacteristics of a chemical reaction, such as:
- time needed to complete the reaction
- physical state of reactants and products
- concentration of each reactant and product
- rate of the reaction
Making a chemical reaction more informative
- Providing the information about catalyst used, temperature and pressure of the reaction above or below the arrow.
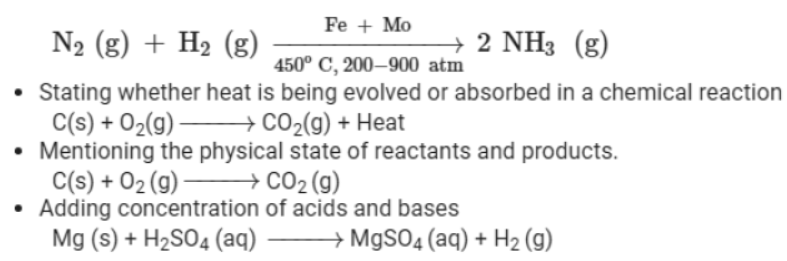
Combination Reactions
You know that chemical changes involve chemical reactions. Chemical reactions are primarily of five types. They are listed as follows:
- Combination reactions
- Decomposition reactions
- Displacement reactions
- Double displacement reactions
- Oxidation and reduction reactions
Here, we will discuss combination reactions in detail. Do you know what actually happens in a combination reaction?
In these reactions, two or more substances combine to form a new compound. The reactants in such reactions can be elements as well as compounds. The general equation used to represent a combination reaction is:
A + Z → AZ
For example, coal is primarily carbon. When it burns, it combines with oxygen present in the air to form carbon dioxide.

Some other examples of combination reactions are discussed below.
- Combination of two elements
On heating, magnesium combines with oxygen present in the air to form magnesium oxide.
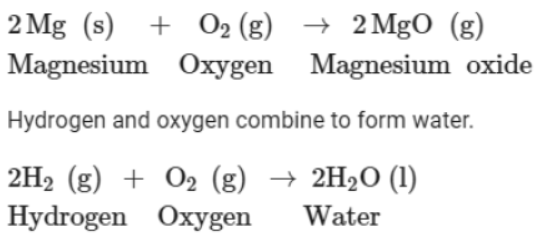
2. Combination of two compounds
Calcium oxide, also known as quick lime, when mixed with water reacts with it to form calcium hydroxide, also known as slaked lime. The chemical equation for the same is given as:
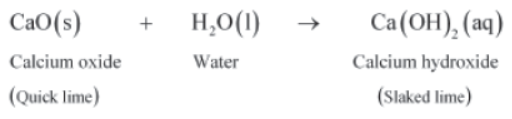
Hence, in combination reactions, two or more compounds combine to produce only one product. Generally, combination reactions are exothermic in nature i.e.
energy is released when two or more compounds combine.
Hence, it can be concluded that combination reactions are generally exothermic in nature. In the above activity, CaO combines with water to give only a single product, Ca (OH)2.
However, there are very few combination reactions which are endothermic in nature. One of the examples of such a reaction is combination of nitrogen and oxygen gas to form nitrogen dioxide gas:

In this reaction, reactants absorb energy from the surroundings in order to form product.
What happens when coal is burned? On burning, coal combines with oxygen to produce carbon dioxide. It also gives a lot of heat energy. Hence, burning of coal is an exothermic reaction.
DO YOU KNOW?
Lime water or slaked lime (Ca(OH)2) is used in white washing of walls. It combines with carbon dioxide present in the air to form a thin layer of calcium carbonate. The chemical formula of calcium carbonate is CaCO3. The chemical equation involved in the reaction can be represented as:
Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O (l)
Slaked lime Carbon dioxide Calcium carbonate Water
Ques. Define a combination reaction. Give one example of a combination reaction which is also exothermic.
Sol: In combination reactions, two or more substances combine to form a new compound.
Only one product is obtained in such reactions. The reactants in such reactions can be elements as well as compounds. The general equation used to represent a combination
reaction is:
A + Z → AZ
For example, calcium oxide reacts vigorously with water to produce calcium hydroxide.
CaO (s) + H2O (l) → Ca(OH)2 (aq)
Calcium oxide Water Calcium hydroxide
A large amount of heat is also evolved during this process, which increases the temperature of the system. Hence, the combination of calcium oxide and water is exothermic in nature.
Decomposition Reactions
We know that chemical reactions are primarily of five types. They are listed as follows:
1. Combination reactions
2. Decomposition reactions
3. Displacement reactions
4. Double displacement reactions
5. Oxidation and reduction reactions
Activity:
Take 3 g of green ferrous sulphate crystals in a dry boiling tube. Heat the boiling tube over the flame of a burner. Observe the change in color of the crystals on heating.
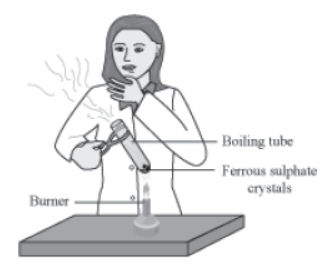
It will be observed that the colour of the crystals undergoes a change. Also, the characteristic smell of burning sulphur is observed. Do you know why this happens?
Here, green crystals of ferrous sulphate lose water on heating. Hence, a change in colour is seen in the crystals. On further heating, it decomposes into ferric oxide, sulphur dioxide, and sulphur trioxide. The chemical equation involved in the reaction can be represented as:
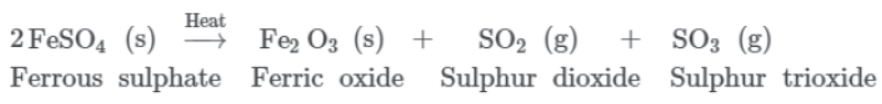
Here, ferrous sulphate breaks down or decomposes to form three new substances. Hence, it is an example of decomposition reactions.
What are decomposition reactions?
In these reactions, a compound breaks down or decomposes to form two or more substances. These reactions are exactly opposite to combination reactions. We know that
there is only one product in combination reactions. Similarly, there is only one reactant in decomposition reactions. The general equation used to represent a decomposition reaction
is:
XY → X + Y
Decomposition reactions require a source of energy in the form of heat, light, or electricity to decompose the compound involved. Hence, these reactions can be classified into three
types, depending on the source of energy for the reaction.
a) Decomposition by heat or thermal decomposition
b) Decomposition by electricity or electrolysis
c) Decomposition by light or photolysis
Let us now study three different types of decomposition reactions.
One of the most common examples of thermal decomposition reactions is the decomposition of calcium carbonate. Calcium carbonate when heated decomposes to form calcium oxide and carbon dioxide.

In this reaction, one compound i.e. calcium carbonate breaks down to form two compounds, namely calcium oxide and carbon dioxide. Hence, it is an example of decomposition
reactions. Commercially, this reaction is very important as calcium oxide (obtained as a product in this reaction) is used in cement and glass industries.
Hands-on Activity
Take about 3 g of solid lead nitrate in a boiling tube. Note the colour of the compound. Heat it in the flame of the Bunsen burner. Observe the change taking place.
You will observe that emission of brown fumes occurs. These fumes are of nitrogen dioxide.
During this reaction, lead nitrate decomposes to form lead oxide, nitrogen dioxide, and oxygen gas. The following reaction takes place:
2Pb(NO3)2 (s) → 2PbO (s) + 4NO2 (g) + O2 (g)
Lead nitrate Lead oxide Nitrogen dioxide Oxygen
The Taj Mahal is made up of marble. Do you know that chemically, marble is nothing but calcium carbonate?

Thermal decomposition of some compounds:
- Metal hydroxides: They decompose on heating to produce metal oxide and water or steam.
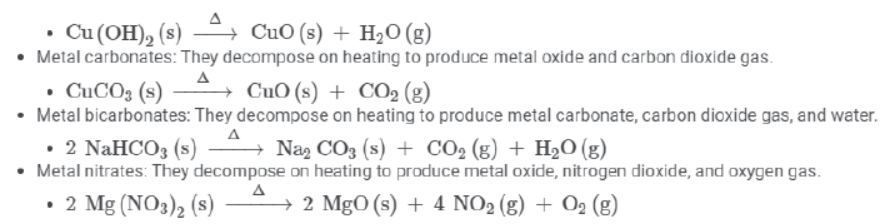
b) Decomposition by electricity
When electricity is passed through water containing a few drops of sulphuric acid, it breaks down to give its constituent elements as products i.e. hydrogen and oxygen. This is known
as electrolysis of water. Let us understand decomposition by electricity with one of its application in the real world.
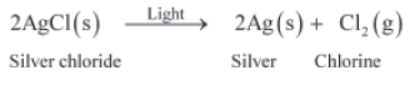
When silver chloride is kept in the sun, it decomposes to form chlorine gas and metallic silver. As the reaction proceeds, the white coloured silver chloride turns grey because of the formation of silver. Chlorine produced in the reaction escapes into the environment as it is produced in the gaseous state.

Figure 3: Photolysis of silver chloride
Silver bromide also undergoes decomposition in a similar manner when exposed to sunlight.
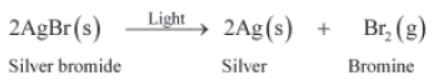
As the above reactions are sensitive to light, they are used in black and white photography.
It is seen that decomposition reactions require a source of energy in the form of heat, light, or electricity to decompose the compound involved. Hence, it can be concluded
that decomposition reactions are endothermic in nature.
Ques.
(a) What is the colour of ferrous sulphate crystals? How does this colour change after heating?
(b) Name the products formed on strongly heating ferrous sulphate crystals.
Sol: a) The colour of ferrous sulphate crystals is green.
On heating, ferrous sulphate crystals (FeSO4.7H2O) lose their water of crystallisation and due to this, the colour of the compound changes to white/colourless.
(b) On strong heating, ferrous sulphate crystals give ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3) as products.

Decomposition reaction occurs in this change.
Ques. Give an example of a decomposition reaction. Describe an activity to illustrate such a reaction by heating.
Sol: Example of decomposition
reaction:

2 g of lead nitrate is taken in a boiling tube and heated. On heating, lead nitrate decomposes to produce lead oxide, nitrogen dioxide, and oxygen. The chemical equation involved in the reaction is:
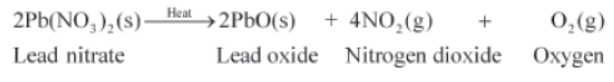
Displacement Reactions
We know that chemical reactions are primarily of five types. They are listed below.
1. Combination reactions
2. Decomposition reactions
3. Displacement reactions
4. Double displacement reactions
5. Oxidation and reduction reactions
In this part, we will discuss displacement and double displacement reactions in detail.
In displacement reactions, a more reactive metal replaces a less reactive metal from the latter’s salt.
Reactions in which a more reactive element replaces a less reactive element from the salt solution of the less reactive element are called displacement reactions.
Do you know that displacement reactions are of two types? They are:
1. Single Displacement Reactions
2. Double Displacement Reactions
Single Displacement Reactions can be better understood with the help of the following figure.

In the above figure, you have three blocks. It will be observed that while red and blue blocks are fixed in, green block is aloof. Now, if a blue block is detached from the red and fixed with the green, it will mean that the green block displaces the red block.
Thus, in a single displacement reaction, an uncombined single element replaces the other element present in a compound.
Another example of single displacement reaction is:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Zinc Copper sulphate Zinc sulphate Copper
The reactivity of metals can be known from the reactivity series, which lists metals in their respective order of reactivity (most reactive at the top, least reactive at the bottom).
Now, consider the following figure.

Do you observe any difference from the first block sequence? In the above figure, there are four different blocks with different colours in two pairs. These blocks are detached.
Then, the blue block is exchanged with the yellow block. This represents a double displacement reaction.
A Double Displacement Reaction is a bimolecular process in which parts of two compounds are exchanged to give two new compounds. The general equation used to represent double displacement reactions can be written as:
AB + CD → AD + BC
Double Displacement Reactions have two common features:
- Firstly, two compounds exchange their ions resulting in the formation of new compounds.
- Secondly, one of the new products formed would be separated from the mixture in some way (commonly as a solid or gas).
Hands on activity
Activity – I
Take 2 mL each of lead nitrate and potassium iodide solution in two separate test tubes. Gently pour the potassium iodide solution into the lead nitrate solution.
As soon as you do this, you will observe the formation of a yellow precipitate. This yellow precipitate is of lead iodide. In this reaction, the two compounds lead nitrate and potassium iodide react by exchanging their ions to form new compounds, lead iodide and potassium nitrate.
The equation involved in this reaction is:

Activity – II
Take five 100 mL beakers and add 20 mL water in them. Label the beakers as I, II, III, IV, and V. Add 5 g copper sulphate to beakers I and II, 5 g zinc sulphate to III and V, and 5 g iron sulphate to beaker IV. Now, add some iron nails to beakers II and V, copper turnings to beakers III and IV, and zinc granules to beaker I. Then, keep the beakers undisturbed for some time and observe carefully.
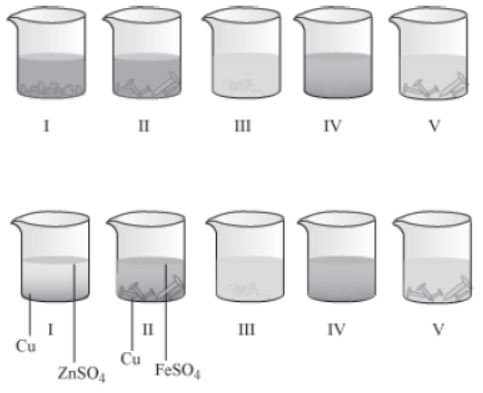
You will observe that the colour of copper sulphate solution changes in beakers I and II. On the other hand, no change is observed in beakers III, IV, and V.
Can you explain these observations using the concept of displacement reactions?
In beaker I, zinc (Zn) replaces copper (Cu) from copper sulphate (CuSO4) solution to form zinc sulphate (ZnSO4) and copper. Because of this, the blue colour of copper sulphate disappears and a reddish brown substance i.e. copper gets deposited at the bottom of the beaker. The chemical equation for the reaction can be
represented as:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Zinc Copper sulphate Zinc sulphate Copper
Similarly, in beaker II, iron replaces copper from copper sulphate solution. Hence, the colour of the solution changes from blue to green and a reddish brown substance gets deposited on the iron nail.
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Iron Copper sulphate Iron(II) sulphate Copper
Do you know why there are no changes in beakers III, IV, and V?
Since no change is observed in beakers III, IV, and V, it can be concluded that copper is less reactive than zinc and iron. Hence, copper can not replace zinc from zinc sulphate solution and iron from iron sulphate solution. Therefore, we can also say that iron is less reactive than zinc.
Hence, iron cannot replace zinc from zinc sulphate solution.
Hence, it can be concluded that in displacement reactions, a more reactive metal replaces a less reactive metal from its salt solution, whereas a less reactive metal cannot replace a more reactive metal.
Types of double displacement reaction: A Double Displacement Reaction is of three types.
Precipitation reaction
In precipitation reaction, soluble ions in separate solutions are mixed together to form an insoluble compound that settles out of the solution as a solid. This insoluble compound is
called a precipitate.
Example:
If an aqueous solution of sodium sulphate is mixed with barium chloride, it will be observed that a white insoluble substance is formed. The white insoluble substance is called a precipitate. Here, barium chloride reacts with sodium sulphate to produce barium sulphate (white insoluble precipitate) and sodium chloride. Thus, this is an example of a
double displacement reaction. The chemical equation involved in the reaction is
BaCl2 (aq) + Na2SO4 (aq) → BaSO4 (s) + 2NaCl (s)
Barium chloride Sodium sulphate Barium sulphate Sodium chloride
Neutralisation reaction
Neutralisation reaction is a chemical reaction in which an acid and a base react to produce salt and water (H2O).
Example:
2NaOH (aq) + H2SO4 (aq) → Na2SO4 (s) + 2H2O (l)
Sodium hydroxide Sulphuric acid Sodium sulphate Water
Gas forming reaction
Gas forming reactions are those reactions in which either, one of the product is formed in gaseous state or a product decomposes instantly to form a gaseous compound.
Example:
2HNO3 (aq) + Na2SO3 (aq) → 2NaNO3 (aq) + H2O (l) + SO2 (g)
Nitric acid Sodium sulphite Sodium nitrate Water Sulphur dioxe
Oxidation and Reduction Reactions
You must have observed that when butter is kept in the open for a long time, it becomes rancid. Also, its smell and taste undergo a change.
Do you know why?
This is because butter undergoes oxidation i.e. it reacts with oxygen and gets oxidized. This process is called rancidity.
What is Oxidation?
Oxidation is defined as a process that involves a gain of oxygen or a loss of hydrogen. When a substance gains oxygen or loses hydrogen during a reaction, it is oxidized.
Let us perform an activity to understand more about these reactions.
Activity:
Take around 1g copper powder (reddish brown in colour) in a china dish and heat it over a burner (as shown in the given figure).
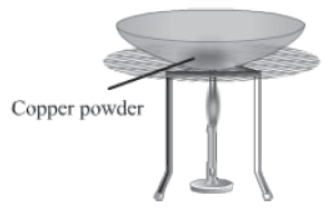
What do you observe?
It will be observed that after some time, the surface of the powder is covered by the layer of a black substance. When copper powder is heated, it combines with oxygen to form copper oxide.
Actually, in the process, copper powder gains oxygen. Thus, it gets oxidized to form copper oxide on heating. This process is called oxidation.

Now, if hydrogen gas is passed over heated copper (II) oxide, then the black coating on the surface turns brown. This is because a reverse reaction takes place and copper is reobtained.
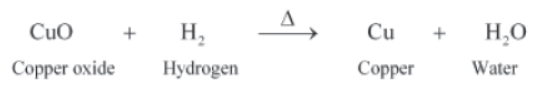
Here, copper (II) oxide loses oxygen and gets reduced to copper. This process is called reduction.
What is Reduction?
Reduction is defined as a process that involves a gain of hydrogen or a loss of oxygen. When a substance loses oxygen or gains hydrogen during a reaction, it is reduced.
Oxidation and reduction always take place simultaneously. Therefore, reactions involving oxidation and reduction are known as Redox (‘Red’ for reduction and ‘ox’ for oxidation) reactions. In a redox reaction, one substance is oxidized, while the other is reduced.
The substances that are reduced (provide oxygen or remove hydrogen) in course of the reaction are called oxidizing agents. These substances oxidise other chemicals in the reaction and are reduced in the process. On the other hand, the substances that are oxidized (remove oxygen or provide hydrogen) are called reducing agents.
For example:
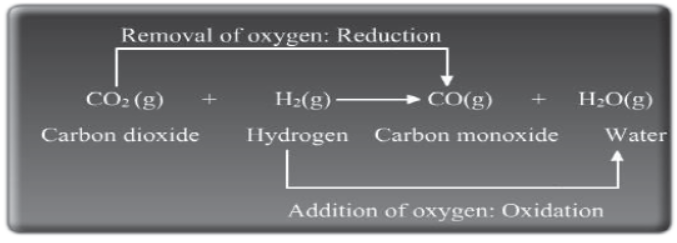
In the above reaction, CO2 gets reduced to CO and here, CO2 is the oxidizing agent. On the other hand, hydrogen gets oxidised to form water and here, H2 is the reducing agent.
We come across many examples of redox reactions in our daily life. For example, in the process of corrosion, metal combines with oxygen and gets corroded. Again, food gets spoiled, when it is oxidised and the process is called rancidity. We will discuss them separately.
Corrosion:
It may be defined as a process where materials, usually metals, are deteriorated because of a chemical reaction with air, moisture, chemicals, etc. For example, corrosion causes damage to car bodies, bridges, iron railings, ships, and all objects made of metals (especially those made from iron). Iron, in the presence of moisture, reacts with oxygen to form iron (III) oxide. This reaction is represented as:

This hydrated iron (III) oxide is rust. If not controlled, rusting can corrode the entire iron present in an object. As rust is softer than iron, the strength of the object decreases when rusting takes place. Every year, a large amount of money is spent on the maintenance of structures made of iron such as bridges, rails, ships etc.
Rancidity:
When fats and oils are oxidized, they become rancid and their smell and taste also change.
Thus, the oxidation of fats and oils can be easily observed by a change in their taste and smell. Oxidation of food can be prevented in many ways. Two common methods are discussed below:
1. Storing food in airtight containers. By doing so, the oxygen available for oxidation becomes limited. Hence, oxidation can be prevented.
2. Sometimes, antioxidants are added to food to prevent their oxidation. These antioxidants are oxidised first, which slows down the process of rancidity. These are reducing agents.
Normally, vitamin C and vitamin E are added as antioxidants.
Ques. What is an oxidation reaction? Give an example of oxidation reaction. Is oxidation an exothermic or an endothermic reaction?
Sol: The reaction in which a substance gains oxygen or loses hydrogen is called an oxidation reaction.
For example, when copper (Cu) is heated in the presence of air, it gets oxidised to form copper oxide (CuO). The chemical equation involved in this reaction can be represented as

Since heat energy is consumed during this process, therefore, this is an endothermic reaction.


