Please refer to Class 12 Chemistry Sample Paper Term 1 Set B with solutions below. The following CBSE Sample Paper for Class 12 Chemistry has been prepared as per the latest pattern and examination guidelines issued by CBSE. By practicing the Chemistry Sample Paper for Class 12 students will be able to improve their understanding of the subject and get more marks.
CBSE Class 12 Chemistry Sample Paper for Term 1
Section ‘A’
1. Bond dissociation enthalpy of E-H (E = element) bond is given below. Which of the compounds will act as the strongest reducing agent ?

(A) NH3
(B) PH3
(C) AsH3
(D) SbH3
Answer
D
2. Most crystals show good cleavage because their atoms, ions or molecules are :
(A) weakly bonded together
(B) strongly bonded together
(C) spherically symmetrical
(D) arranged in planes.
Answer
D
3. The increase in the temperature of the aqueous solution will result in its :
(A) Molarity to increase
(B) Molarity to decrease
(C) Mole fraction to increase
(D) Mass % to increase
Answer
B
4. Copper crystallizes in face-centred cubic lattice with a unit cell length of 361 pm. What is the radius of copper atom in pm ?
(A) 157
(B) 181
(C) 108
(D) 128
Answer
D
5. The IUPAC name of anisole is :
(A) 2-methyltoluene
(B) Methyl phenyl ether
(C) Methoxybenzene
(D) Ethoxybenzene
Answer
C
6. As compared to alkyl halides, aryl halides are less reactive towards nucleophilic substitution because of :
(A) inductive effect
(B) larger carbon -halogen bond
(C) resonance stabilisation
(D) formation of less stable carbonium ion.
Answer
C
7. Choose the one which is most reactive towards nucleophilic substitution :
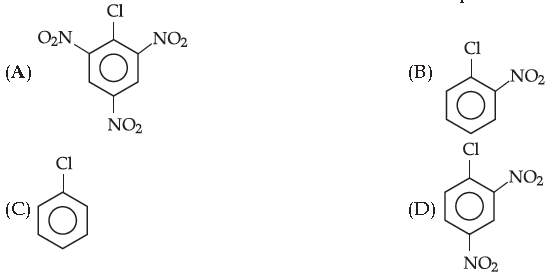
Answer
A
8. Choose the one which will show fastest reaction with aqueous NaOH ?

Answer
B
9. Arrange the following compounds in increasing order of boiling point :
Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
(A) Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol
(B) Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
(C) Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol
(D) Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol
Answer
A
10. The correct statement regarding defects in crystalline solids is :
(A) Frenkel defects decrease the density of crystalline solids
(B) Frenkel defect is a dislocation defect
(C) Frenkel defect is found in halides of alkaline metals
(D) Schottky defects have no effect on the density of crystalline solids.
Answer
B
11. Which chloro derivative of benzene would readily hydrolyse with aq. sodium hydroxide to yield hydroxy derivative ?
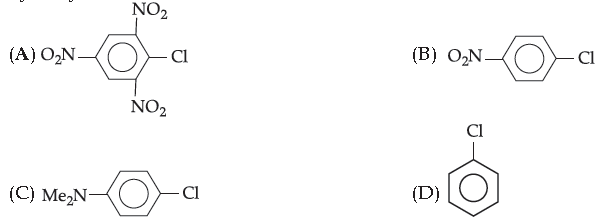
Answer
A
12. Schottky defect in crystals is observed when :
(A) density of the crystals is increased
(B) unequal number of cations and anions are missing from the lattice
(C) an ion leaves its normal site and occupies an interstitial site
(D) equal number of cations and anions are missing from the lattice.
Answer
D
13. Phenols do not undergo nucleophilic substitution because :
(i) —OH group in phenol is strong electron donating.
(ii) Benzene ring repels nucleophile.
(iii) —OH group is strong electron accepting group.
Which of the above is the correct answer :
(A) Only (i)
(B) Only (ii)
(C) (i) and (ii)
(D) (ii) and (iii)
Answer
D
14. The increasing reactivity of the compounds
(i) MeBr (ii) PhCH2Br
(iii) MeCl and (iv) p-MeOC6H4 Br is :
(A) (i)< (ii) <(iii) < (iv)
(B) (iv) < (iii) < (i) < (ii)
(C) (i)<(iii) < (iv) < (ii)
(D) (iv) < (i) < (iii) <(ii)
Answer
B
15. Which of the following is not an example of denaturation of proteins :
(A) boiled eggs become hard
(B) boiled corns
(C) cooked meat becomes firm
(D) curdling of milk
Answer
B
16. In the given reaction, the major product formed is :
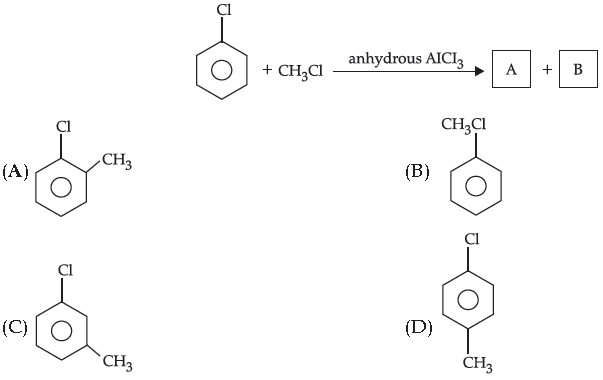
Answer
D
17. The boiling point of ethanol is higher than ether due to :
(A) Presence of-OH group
(B) Presence of-O-group
(C) Intermolecular hydrogen bonding
(D) High molecular weight
Answer
C
18. Affinity for hydrogen decreases in the group from fluorine to iodine. Which of the halogen acids should have highest bond dissociation enthalpy ?
(A) H-F
(B) HCl
(C) HBr
(D) HI
Answer
A
19. If two liquids A and B form minimum boiling azeotrope at some specific composition then _________.
(A) A–B interactions are stronger than those between A–A or B–B.
(B) Vapour pressure of solution increases because more number of molecules of liquids A and B can escape from the solution.
(C) Vapour pressure of solution decreases because less number of molecules of only one of the liquids escape from the solution.
(D) A–B interactions are weaker than those between A–A or B–B.
Answer
D
20. Reduction potentials of some ions are given below. Arrange them in the decreasing order of oxidisingpower.
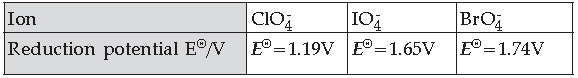
(A) ClO–4 > IO–4 > BrO–4
(B) IO–4 > BrO–4 > ClO–4
(C) BrO–4 > IO–44 > ClO–4
(D) BrO–4 > ClO–4 > IO–4
Answer
B
21. What is the correct function of DNA :
(A) Protein synthesis
(B) They help to maintain the balance of biological activities in the body.
(C) They help to increase the glucose level in the blood
(D) They help in synthesis of enzymes
Answer
A
22. Which one of the following does not exist ?
(A) XeOF4
(B) NeF2
(C) XeF2
(D) XeF6
Answer
B
23. What is formed when a primary alcohol undergoes catalytic dehydrogenation ?
(A) Aldehyde
(B) Ketone
(C) Alkene
(D) Acid
Answer
A
24. IUPAC name for the given compound is :
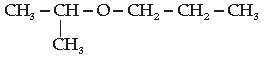
(A) 2-ethoxy-2-methylethane
(B) 2-propoxypropane
(C) 2-methyl-2-ethoxypropane
(D) None of the above
Answer
B
25. Value of Henry’s constant KH is ________________.
(A) Increases with increase in temperature.
(B) Decreases with increase in temperature
(C) Remains constant
(D) First increases then decreases.
Answer
A
Section ‘B’
26. On heating glycerol with conc. H2SO4, a compound is obtained which has bad odour. The compound is :
(A) acrolein
(B) formic acid
(C) allyl alcohol
(D) glycerol sulphate
Answer
A
27. A compound of molecular formula C7H16 shows optical isomerism, compound will be :
(A) 2, 3-dimethylpentane
(B) 2, 2-dimethylbutane
(C) 2-methylhexane
(D) None of these
Answer
A
28. Which of the following orders are correct as per the properties mentioned against each ?

Answer
A
29. For a dilute solution containing 2.5 g of a non-volatile non-electrolyte solute in 100 g of water, the elevation in boiling point at 1 atm pressure is 2°C. Assuming concentration of solute is much lower than the concentration of solvent, the vapour pressure (mm of Hg) of the solution is (take Kb = 0.76 K kg mol–1)
(A) 724
(B) 740
(C) 736
(D) 718
Answer
C
30. HBr reacts fastest with :
(A) 2-Methylpropan-1-ol
(B) Methylprpan-2-ol
(C) Propane-2-ol
(D) Propan-1-ol.
Answer
B
31. Which of the following statements are true ?
(A) Only type of interactions between particles of noble gases are due to weak dispersion forces.
(B) Ionisation enthalpy of molecular oxygen is very close to that of xenon.
(C) Hydrolysis of XeF6 is a redox reaction.
(D) Xenon fluorides are not reactive.
Answer
A
32. Hot conc. H2SO4 acts as moderately strong oxidizing agent. It oxidizes both metals and non-metals.
Which of the following element is oxidized by conc. H2SO4 into two gaseous products ?
(A) Cu
(B) S
(C) C
(D) Zn
Answer
C
33. When phenol is treated with excess bromine water. It gives :
(A) m-bromophenol
(B) o-and p-bromophenols
(C) 2, 4-dibromophenol
(D) 2, 4, 6-tribromophenol
Answer
D
34. Which one of the following pairs represents stereoisomerism ?
(A) Chain isomerism and rotational isomerism
(B) Structural isomerism and geometrical isomerism
(C) Linkage isomerism and geometrical isomerism
(D) Optical isomerism and geometrical isomerism
Answer
D
35. Which one of the following molecular hydrides acts as a Lewis acid ?
(A) NH3
(B) H2O
(C) B2H6
(D) CH4
Answer
C
36. Which one of the following statements is not true regarding (+) lactose ?
(A) On hydrolysis (+) lactose gives equal amount of D(+) glucose and D(+) galactose
(B) (+) Lactose is a β-glucoside formed by the union of a molecule of D(+) glucose and a moleculeof D(+) galactose
(C) (+) Lactose is a reducing sugar and does not exhibit mutarotation.
(D) (+) Lactose, C12H22O11 contains 8-OH groups
Answer
C
37. The straight chain polymer is formed by :
(A) Hydrolysis of (CH3)3SiCl followed by condensation polymerisation
(B) Hydrolysis of CH3SiCl3 followed by condensation polymerisation
(C) Hydrolysis of (CH3)4Si by addition polymerisation
(D) Hydrolysis of (CH3)2SiCl2 followed by condensation polymerisation
Answer
D
38. Ionic solids, with Schottky defects, contain in their structure :
(A) cation vacancies only
(B) cation vacancies and interstitial cations
(C) equal number of cation and anion vacancies
(D) anion vacancies and interstitial anions
Answer
C
39. The basic structural unit of silicates :
(A) SiO–
(B) SiO44–
(C) SiO32–
(D) SiO42–
Answer
B
40. The heating of phenyl-methyl ethers with HI produces :
(A) ethyl chlorides
(B) iodobenzene
(C) phenol
(D) benzene
Answer
C
41. Among the following sets of reactants which one produces anisole ?
(A) CH3CHO, RMgX
(B) C6H5OH, NaOH, CH3I
(C) C6H5OH, neutral FeCl3
(D) C6H5CH3, CH3COCl, AlCl3
Answer
B
42. Choose the statement which is incorrect to explain – Noble gases are chemically inactive :
(A) They have completely filled valence shell.
(B) They have large electron gain enthalpy values.
(C) They have high ionization enthalpy.
(D) They have low ionization enthalpy
Answer
D
43. Ethanol and dimethyl ether form a pair of functional isomers. The boiling point of ethanol is higher
than that of dimethyl ether, due to the presence of :
(A) H-bonding in ethanol
(B) H-bonding in dimethyl ether
(C) CH3 group is ethanol
(D) CH3 group in dimethyl ether
Answer
A
44. Chlorobenzene reacts with Mg in dry ether to give a compound (A) which further reacts with ethanol to yield :
(A) Phenol
(B) Benzene
(C) Ethyl benzene
(D) Phenyl ether.
Answer
B
From Q.45 to Q.49, Given below are two statements labelled as Assertion (A) and Reason (R) and at the end of each question give the following line select the most appropriate answers from the options given below :
(A) Both A and R are true and R is the correct explanation of A.
(B) Both A and R are true but R is NOT the correct explanation of A.
(C) A is true but R is false.
(D) A is false and R is true.
45. Assertion(A) : Group 18 gases exhibit very high ionisation enthalpy.
Reason (R) : They have a stable electronic configuration.
Answer
A
46. Assertion (A) : The stereoisomers related to each other as non-superimposable mirror images are called enantiomers.
Reason (R) : Enantiomers possess identical physical properties.
Answer
B
47. Assertion (A) : NaCl reacts with concentrated H2SO4 to give colourless fumes with pungent smell. But on adding MnO2 the fumes become greenish yellow.
Reason (R) : MnO2 oxidizes HCl to chlorine gas which is greenish yellow.
Answer
A
48. Assertion (A) : SF6 cannot be hydrolysed but SF4 can be.
Reason (R) : Six atoms in SF6 prevent the attack of H2O on sulphur atom of SF6.
Answer
A
49. Assertion (A) : Aquatic species are more comfortable in cold waters rather than in warm waters.
Reason (R) : The solubility of gases decreases with decrease of temperature.
Answer
C
Section ‘C’
50. Choose the incorrect statement :
(A) A racemic mixture is optically active
(B) A racemic mixture is optically inactive.
(C) A racemic mixture has two enantiomers in equal proportion.
(D) A racemic mixture has zero optical rotation.
Answer
A
51. The correct order regarding the electronegativity of hybrid orbitals of carbon is :
(A) sp < sp2 < sp3
(B) sp < sp2 < sp3
(C) sp > sp2 > sp3
(D) sp < sp2 > sp3
Answer
C
52. Which one is formed when sodium phenoxide is heated with ethyl iodide ?
(A) Phenetole
(B) Ethyl phenyl alcohol
(C) Phenol
(D) None of these
Answer
A
CASE 1 : Read the passage given below and answer the following questions 53-55
II. Read the passage given below and answer the following questions :
In Ideally ionic structures, the coordination numbers of the ions are determined by electrostatic considerations. Cations surround themselves with as many anions as possible and vice versa. This maximizes the attractions between neighbouring ions of opposite charge and hence maximizes the lattice energy of the crystal. This requirement led to the formulation of the radius ratio rule for ionic structures in which the ions and the structure adopted for a particular compound depend on the relative sizes of the ions. Thus, for the stable ionic crystalline structures, there is definite radius ratio limit for a cation to fit perfectly in the lattice of anions ralled radius ratio rule. This depends upon the ratio of radii of two types of ions. r+/r_
This ratio for coordination numbers 3.4,6 and 8 are respectively 0.155 – 0.225,0.225 – 0.414,0.414 -0.732 and 0.732 – 1.000. The coordination number of ionic solids also depends upon temperature and pressure. On applying high pressure, coordination number increases. On the other hand, on applying high temperature, it decreases.
53. Choose the correct two -dimensional co-ordination number of a molecule in a square close packed
layer :
(A) 2
(B) 4
(C) 6
(D) 8
Answer
B
54. If the radius of Na+ ion is 95 pm and that of Cl– ion is 181pm, the coordination number of Na+ ion is :
(A) 6
(B) 4
(C) 8
(D) 12
Answer
A
55. The coordination number in hcp is :
(A) 6
(B) 12
(C) 18
(D) 24
Answer
B
