Please refer to the Matter In Our Surroundings Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 9th Science book. We have provided chapter wise Notes for Class 9 Science as per the latest examination pattern.
Revision Notes Chapter 1 Matter In Our Surroundings
Students of Class 9 Science will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 9 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
KEY CONCEPTS :
Pre requisites
- Definition of matter.
- Elementary idea of three physical states of matter .
SURVEY ANALYSIS
Conceptual levels of comprehension on the basis of feedback taken from the students

- Particle Nature of Matter
- Anything that occupies space and has mass and is felt by senses is called matter.
- Matter is the form of five basic elements the Panch tatva – air , earth ,fire , sky and water.
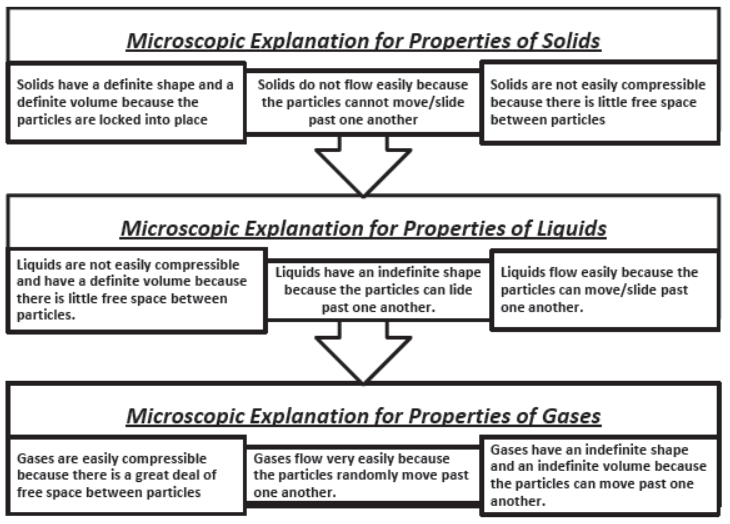
Characteristics of particles of matter
- Made of tiny particles.
- Vacant spaces exist in particles.
- Particles are in continuous motion.
- Particles are held together by forces of attraction.
Q.1 Define matter.
Q.2 What happens if you put copper sulphate crystals in water?
2. States of Matter
Basis of Classification of Types
- Based upon particle arrangement
- Based upon energy of particles
- Based upon distance between particles
Five states of matter
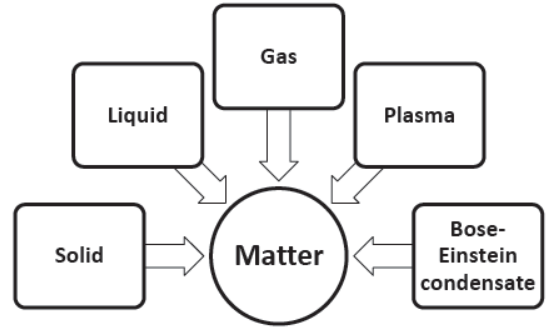

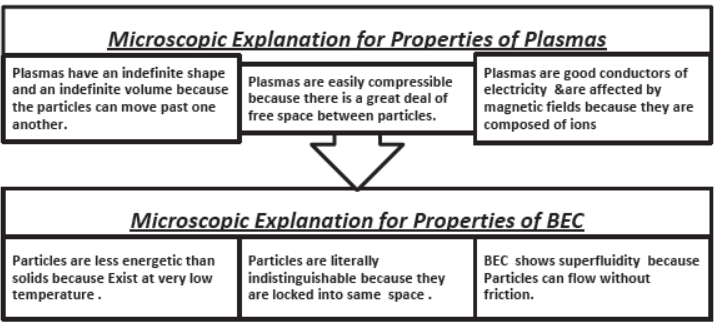
(iv) Plasma (non –evaluative)

(v) Bose-Einstein condensate (non –evaluative)

(non –evaluative)↓
3. Interchange in states of matter
Matter Can Change its State
Water can exist in three states of matter –
• Solid, as ice ,
• Liquid, as the familiar water, and
• Gas, as water vapour.
Sublimation : The changing of solid directly into vapours on heating & vapours into solid on cooling. Ex. Ammonium chloride , camphor & iodine.
a) Effect of change in temperature
The temperature effect on heating a solid varies depending on the nature of the solid & the conditions required in bringing the change .
- On increasing the temperature of solids, the kinetic energy of the particles increases which overcomes the forces of attraction between the particles thereby solid melts and is converted to a liquid.
- The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
- The melting point of ice is 273.16 K.
- The process of melting, that is, change of solid state into liquid state is also known as fusion.
b) Effect of Change of Pressure
- Increasing or decreasing the pressure can change the state of matter. Applying pressure and reducing temperature can liquefy gases.
- Solid carbon dioxide (CO2) is stored under high pressure. Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without cominginto liquid state. This is the reason that solid carbon dioxide is also known as dry ice.
Latent Heat :
The hidden heat which breaks the force of attraction between the molecules during change of state.
| Fusion | Vaporisation |
| Heat energy required to change 1kg of solid into liquid. | Heat energy required to change 1kg of liquid to gas at atmospheric pressure at its boiling point. |
4. Evaporation & Boiling
- Particles of matter are always moving and are never at rest.
- At a given temperature in any gas, liquid or solid, there are particles with different amounts of kinetic energy.
- In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour .
- This phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation.
⇒ Factors Affecting Evaporation
- The rate of evaporation increases with an increase of surface area.
- With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
- Humidity is the amount of water vapour present in air. The air around us cannot hold more than a definite amount of water vapour at a given temperature. If the amount of water in air is already high, the rate of evaporation decreases.
- Wind speed : the higher the wind speed , the more evaporation.
Evaporation cause cooling
The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation,
Evaporation Vs Boiling
- Boiling is a bulk phenomenon. Particles from the bulk (whole) of the liquid change into vapour state.
- Evaporation is a surface phenomenon. Particles from the surface gain enough energy to overcome the forces of attraction present in the liquid and change into the vapour state.
5. Kelvin & Celsius Scale
- Kelvin is the SI unit of temperature, 00 C =273.16 K. we take 00 C = 273 K.
- SI unit of temperature is Kelvin. T (K)= T (oC) +273.
- Kelvin scale of temperature has always positive sign , hence regarded as better scale than Celsius.
- Atmosphere (atm) is a unit of measuring pressure exerted by a gas. The SI unit of pressure is Pascal (Pa):
- 1 atmosphere = 1.01 × (10 to the power 5) Pa. The pressure of air in atmosphere is called atmospheric pressure. The atmospheric pressure at sea level is 1 atmosphere, and is taken as the normal atmospheric pressure.