Please refer to Atoms MCQ Questions Class 12 Physics below. These MCQ questions for Class 12 Physics with answers have been designed as per the latest NCERT, CBSE books, and syllabus issued for the current academic year. These objective questions for Atoms will help you to prepare for the exams and get more marks.
Atoms MCQ Questions Class 12 Physics
Please see solved MCQ Questions for Atoms in Class 12 Physics. All questions and answers have been prepared by expert faculty of standard 12 based on the latest examination guidelines.
MCQ Questions Class 12 Physics Atoms
Question. An alpha nucleus of energy (1/2)mv2 bombards a heavy nuclear target of charge Ze. Then the distance of closest approach for the alpha nucleus will be proportional to
(a) 1/Ze
(b) v2
(c) 1/m
(d) 1/v4
Answer
C
Question. In a Rutherford scattering experiment when a projectile of charge z1 and mass M1 approaches a target nucleus of charge z2 and mass M2, the distance of closest approach is r0. The energy of the projectile is
(a) directly proportional to z1z2
(b) inversely proportional to z1
(c) directly proportional to mass M1
(d) directly proportional to M1 × M2
Answer
A
Question. The wavelength of the first line of Lyman series for hydrogen atom is equal to that of the second line of Balmer series for a hydrogen like ion. The atomic number Z of hydrogen like ion is
(a) 3
(b) 4
(c) 1
(d) 2
Answer
D
Question. The total energy of an electron in an atom in an orbit is – 3.4 eV. Its kinetic and potential energies are, respectively
(a) 3.4 eV, 3.4 eV
(b) – 3.4 eV, – 3.4 eV
(c) – 3.4 eV, – 6.8 eV
(d) 3.4 eV, – 6.8 eV
Answer
D
Question. Which source is associated with a line emission spectrum?
(a) Electric fire
(b) Neon street sign
(c) Red traffic light
(d) Sun
Answer
B
Question. An electron is moving round the nucleus of a hydrogen atom in a circular orbit of radius r. The Coulomb force F→ between the two is

Answer
D
Question. Ratio of longest wavelengths corresponding to Lyman and Balmer series in hydrogen spectrum is
(a) 7/29
(b) 9/31
(c) 5/27
(d) 5/23
Answer
C
Question. For which one of the following, Bohr model is not valid?
(a) Hydrogen atom
(b) Singly ionised helium atom (He+)
(c) Deuteron atom
(d) Singly ionised neon atom (Ne+)
Answer
D
Question. When an a-particle of mass m moving with velocity v bombards on a heavy nucleus of charge Ze, its distance of closest approach from the nucleus depends on m as
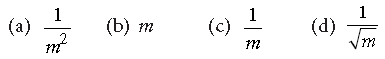
Answer
C
Question. The radius of the first permitted Bohr orbit for the electron, in a hydrogen atom equals 0.51 Å and its ground state energy equals –13.6 eV. If the electron in the hydrogen atom is replaced by muon (μ–) [charge same as electron and mass 207 me], the first Bohr radius and ground state energy will be
(a) 0.53 × 10–13 m, –3.6 eV
(b) 25.6 × 10–13 m, –2.8 eV
(c) 2.56 × 10–13 m, –2.8 keV
(d) 2.56 × 10–13 m, –13.6 eV
Answer
C
Question. An electron in the hydrogen atom jumps from excited state n to the ground state. The wavelength so emitted illuminates a photosensitive material having work function 2.75 eV. If the stopping potential of the photoelectron is 10 V, then the value of n is
(a) 2
(b) 3
(c) 4
(d) 5
Answer
C
Question. The ratio of wavelengths of the last line of Balmer series and the last line of Lyman series is
(a) 1
(b) 4
(c) 0.5
(d) 2
Answer
B
Question. The electron in the hydrogen atom jumps from excited state (n = 3) to its ground state (n = 1) and the photons thus emitted irradiate a photosensitive material. If the work function of the material is 5.1 eV, the stopping potential is estimated to be (the energy of the electron in nth state En = −13.6/n2,. eV )
(a) 5.1 V
(b) 12.1 V
(c) 17.2 V
(d) 7 V
Answer
D
Question. Given the value of Rydberg constant is 107 m–1, the wave number of the last line of the Balmer series in hydrogen spectrum will be
(a) 0.25 × 107 m–1
(b) 2.5 × 107 m–1
(c) 0.025 × 104 m–1
(d) 0.5 × 107 m–1
Answer
A
Question. Out of the following which one is not a possible energy for a photon to be emitted by hydrogen atom according to Bohr’s atomic model?
(a) 0.65 eV
(b) 1.9 eV
(c) 11.1 eV
(d) 13.6 eV
Answer
C
Question. The ratio of kinetic energy to the total energy of an electron in a Bohr orbit of the hydrogen atom, is
(a) 1 : 1
(b) 1 : –1
(c) 2 : –1
(d) 1 : –2
Answer
B
Question. Consider 3rd orbit of He+ (Helium), using non-relativistic approach, the speed of electron in this orbit will be [given K = 9 × 109 constant, Z = 2 and h (Planck’s constant) = 6.6 × 10–34 J s]
(a) 0.73 × 106 m/s
(b) 3.0 × 108 m/s
(c) 2.92 × 106 m/s
(d) 1.46 × 106 m/s
Answer
D
Question. The energy of a hydrogen atom in the ground state is –13.6 eV. The energy of a He+ ion in the first excited state will be
(a) –13.6 eV
(b) –27.2 eV
(c) –54.4 eV
(d) –6.8 eV
Answer
A
Question. The ground state energy of hydrogen atom is –13.6 eV. When its electron is in the first excited state, its excitation energy is
(a) 10.2 eV
(b) 0
(c) 3.4 eV
(d) 6.8 eV
Answer
A
Question. An electron in hydrogen atom makes a transition n1 → n2 where n1 and n2 are principal quantum numbers of the two states. Assuming Bohr’s model to be valid, the time period of the electron in the initial state is eight times that in the final state. The possible values of n1 and n2 are
(a) n1 = 6 and n2 = 2
(b) n1 = 8 and n2 = 1
(c) n1 = 8 and n2 = 2
(d) n1 = 4 and n2 = 2
Answer
D
Question. Monochromatic radiation emitted when electron on hydrogen atom jumps from first excited to the ground state irradiates a photosensitive material. The stopping potential is measured to be 3.57 V. The threshold frequency of the material is
(a) 4 × 1015 Hz
(b) 5 × 1015 Hz
(c) 1.6 × 1015 Hz
(d) 2.5 × 1015 Hz
Answer
C
Question. The total energy of electron in the ground state of hydrogen atom is –13.6 eV. The kinetic energy of an electron in the first excited state is
(a) 6.8 eV
(b) 13.6 eV
(c) 1.7 eV
(d) 3.4 eV
Answer
D
Question. The total energy of an electron in the first excited state of hydrogen atom is about –3.4 eV. Its kinetic energy in this state is
(a) 3.4 eV
(b) 6.8 eV
(c) –3.4 eV
(d) –6.8 eV
Answer
A
Question. The ionization energy of hydrogen atom is 13.6 eV. Following Bohr’s theory, the energy corresponding to a transition between 3rd and 4th orbit is
(a) 3.40 eV
(b) 1.51 eV
(c) 0.85 eV
(d) 0.66 eV
Answer
D
Question. The Bohr model of atoms
(a) Assumes that the angular momentum of electrons is quantized.
(b) Uses Einstein’s photoelectric equation.
(c) Predicts continuous emission spectra for atoms.
(d) Predicts the same emission spectra for all types of atoms.
Answer
A
Question. In which of the following systems will the radius of the first orbit (n = 1) be minimum?
(a) doubly ionized lithium
(b) singly ionized helium
(c) deuterium atom
(d) hydrogen atom
Answer
A
Question. When hydrogen atom is in its first excited level, its radius is ………. of the Bohr radius.
(a) twice
(b) 4 times
(c) same
(d) half
Answer
B
Question. According to Bohr’s principle, the relation between principal quantum number (n) and radius of orbit (r) is

Answer
D
Question. In the Bohr model of a hydrogen atom, the centripetal force is furnished by the coulomb attraction between the proton and the electron. If a0 is the radius of the ground state orbit, m is the mass and e is the charge on the electron and e0 is the vacuum permittivity, the speed of the electron is
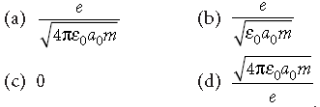
Answer
A
Question. The energy of the ground electronic state of hydrogen atom is –13.6 eV. The energy of the first excited state will be
(a) –27.2 eV
(b) –52.4 eV
(c) –3.4 eV
(d) –6.8 eV
Answer
C
Question. When a hydrogen atom is raised from the ground state to an excited state,
(a) both K.E. and P.E. increase
(b) both K.E. and P.E. decrease
(c) the P.E. decreases and K.E. increases
(d) the P.E. increases and K.E. decreases.
Answer
D
Question. In terms of Bohr radius a0, the radius of the second Bohr orbit of a hydrogen atom is given by
(a) 4a0
(b) 8a0
(c) √2a0
(d) 2a0
Answer
A
Question. Hydrogen atom in ground state is excited by a monochromatic radiation of λ = 975 Å. Number of spectral lines in the resulting spectrum emitted will be
(a) 3
(b) 2
(c) 6
(d) 10
Answer
C
Question. The energy of hydrogen atom in nth orbit is En then the energy in nth orbit of singly ionised helium atom will be
(a) 4En
(b) En/4
(c) 2En
(d) En/2
Answer
A
Question. The life span of atomic hydrogen is
(a) fraction of one second
(b) one year
(c) one hour
(d) one day
Answer
A
Question. Electron in hydrogen atom first jumps from third excited state to second excited state and then from second excited to the first excited state. The ratio of the wavelengths λ1 : λ2 emitted in the two cases is
(a) 7/5
(b) 27/20
(c) 27/5
(d) 20/7
Answer
D
Question. The ground state energy of H-atom is –13.6 eV. The energy needed to ionize H-atom from its second excited state
(a) 1.51 eV
(b) 3.4 eV
(c) 13.6 eV
(d) none of these
Answer
A
Question. If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd orbit, it emits a photon of wavelength λ. When it jumps from the 4th orbit to the 3rd orbit, the corresponding wavelength of the photon will be
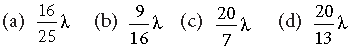
Answer
C
Question. The ionization energy of the electron in the hydrogen atom in its ground state is 13.6 eV. The atoms are excited to higher energy levels to emit radiations of 6 wavelengths. Maximum wavelength of emitted radiation corresponds to the transition between
(a) n = 3 to n = 1 states
(b) n = 2 to n = 1 states
(c) n = 4 to n = 3 states
(d) n = 3 to n = 2 states
Answer
C
Question. To explain his theory, Bohr used
(a) conservation of linear momentum
(b) quantisation of angular momentum
(c) conservation of quantum frequency
(d) none of these
Answer
B
Question. The ionisation energy of hydrogen atom is 13.6 eV, the ionisation energy of a singly ionised helium atom would be
(a) 13.6 eV
(b) 27.2 eV
(c) 6.8 eV
(d) 54.4 eV
Answer
D
Question. Ionization potential of hydrogen atom is 13.6 eV. Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy 12.1 eV. According to Bohr’s theory, the spectral lines emitted by hydrogen will be
(a) one
(b) two
(c) three
(d) four.
Answer
C
Question. The interplanar distance in a crystal is 2.8 × 10–8 m. The value of maximum wavelength which can be diffracted
(a) 2.8 × 10–8 m
(b) 5.6 × 10–8 m
(c) 1.4 × 10–8 m
(d) 7.6 × 10–8 m
Answer
B
Question. The minimum wavelength of the X-rays produced by electrons accelerated through a potential difference of V volts is directly proportional to
(a) 1/√V
(b) 1/V
(c) √V
(d) V2
Answer
B
Question. Energy levels A, B and C of a certain atom corresponding to increasing values of energy i.e., EA < EB < EC. If λ1, λ2 and λ3 are wavelengths of radiations corresponding to transitions C to B, B to A and C to A respectively, which of the following relations is correct?

Answer
B
Question. Maximum frequency of emission is obtained for the transition
(a) n = 2 to n = 1
(b) n = 6 to n = 2
(c) n = 1 to n = 2
(d) n = 2 to n = 6
Answer
A
Question. An electron of a stationary hydrogen atom passes from the fifth energy level to the ground level. The velocity that the atom acquired as a result of photon emission will be
(a) 24hR/25m
(b) 25hR/24m
(c) 25m/24hR
(d) 24m/25hR
Answer
A
Question. Hydrogen atoms are excited from ground state of the principle quantum number 4. Then the number of spectral lines observed will be
(a) 3
(b) 6
(c) 5
(d) 2
Answer
B
Question. Consider an electron in the nth orbit of a hydrogen atom in the Bohr model. The circumference of the orbit can be expressed in terms of de Broglie wavelength λ of that electron as
(a) (0.529)nλ
(b) √nλ
(c) (13.6)λ
(d) nλ
Answer
D
Question. The transition from the state n = 3 to n = 1 in a hydrogen like atom results in ultraviolet radiation. Infrared radiation will be obtained in the transition from
(a) 2 → 1
(b) 3 → 2
(c) 4 → 2
(d) 4 → 3
Answer
D
Question. When an electron does transition from n = 4 to n = 2, then emitted line spectrum will be
(a) first line of Lyman series
(b) second line of Balmer series
(c) first line of Paschen series
(d) second line of Paschen series.
Answer
B
Question. The figure indicates the energy level diagram of an atom and the origin of six spectral lines in emission (e.g., line no. 5 arises from the transition from level B to A).
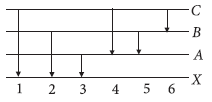
Which of the following spectral lines will occur in the absorption spectrum?
(a) 4, 5, 6
(b) 1, 2, 3, 4, 5, 6
(c) 1, 2, 3
(d) 1, 4, 6
Answer
C
Question. The figure represents the observed intensity of X-rays emitted by an X-ray tube,1 as a function of wavelength. The sharp peaks A and B denote
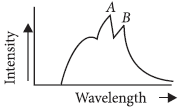
(a) white radiations
(b) characteristic radiations
(c) band spectrum
(d) continuous spectrum
Answer
B
Question. An electron makes a transition from orbit n = 4 to the orbit n = 2 of a hydrogen atom. What is the wavelength of the emitted radiations?
(R = Rydberg’s constant)
(a) 16/4R
(b) 16/5R
(c) 16/2R
(d) 16/3R
Answer
D



