Please refer to Nuclei MCQ Questions Class 12 Physics below. These MCQ questions for Class 12 Physics with answers have been designed as per the latest NCERT, CBSE books, and syllabus issued for the current academic year. These objective questions for Nuclei will help you to prepare for the exams and get more marks.
Nuclei MCQ Questions Class 12 Physics
Please see solved MCQ Questions for Nuclei in Class 12 Physics. All questions and answers have been prepared by expert faculty of standard 12 based on the latest examination guidelines.
MCQ Questions Class 12 Physics Nuclei
Question. The constituents of atomic nuclei are believed to be
(a) neutrons and protons
(b) protons only
(c) electrons and protons
(d) electrons, protons and neutrons
Answer
A
Question. A nucleus represented by the symbol AZX has
(a) Z neutrons and A – Z protons
(b) Z protons and A – Z neutrons
(c) Z protons and A neutrons
(d) A protons and Z – A neutrons
Answer
B
Question. The mass number of a nucleus is
(a) always less than its atomic number
(b) always more than its atomic number
(c) sometimes equal to its atomic number
(d) sometimes less than and sometimes more than its atomic number
Answer
C
Question. Two nuclei have their mass numbers in the ratio of 1 : 3. The ratio of their nuclear densities would be
(a) (3)1/3 : 1
(b) 1 : 1
(c) 1 : 3
(d) 3 : 1
Answer
B
Question. If the nucleus 1327Al has a nuclear radius of about 3.6 fm, then 32125Te would have its radius approximately as
(a) 9.6 fm
(b) 12.0 fm
(c) 4.8 fm
(d) 6.0 fm
Answer
D
Question. In the nucleus of 11Na23, the number of protons, neutrons and electrons are
(a) 11, 12, 0
(b) 23, 12, 11
(c) 12, 11, 0
(d) 23, 11, 12
Answer
A
Question. Which one of the following pairs of nuclei are isotones?
(a) 34Se74, 31Ga71
(b) 38Sr84, 38Sr86
(c) 42Mo92, 40Zr92
(d) 20Ca40, 16S32
Answer
A
Question. Atomic weight of Boron is 10.81 and it has two isotopes 5B10 and 5B11. Then the ratio of 5B10 : 5B11 in nature would be
(a) 15 : 16
(b) 10 : 11
(c) 19 : 81
(d) 81 : 19
Answer
C
Question. The nuclei 6C13 and 7N14 can be described as
(a) isotones
(b) isobars
(c) isotopes of carbon
(d) isotopes of nitrogen
Answer
A
Question. If the nuclear radius of 27Al is 3.6 fermi, the approximate nuclear radius of 64Cu in fermi is
(a) 2.4
(b) 1.2
(c) 4.8
(d) 3.6
Answer
C
Question. The mass number of He is 4 and that of sulphur is 32. The radius of sulphur nucleus is larger than that of helium by the factor of
(a) 4
(b) 2
(c) √8
(d) 8
Answer
B
Question. The energy required to break one bond in DNA is 10–20 J. This value in eV is nearly
(a) 6
(b) 0.6
(c) 0.06
(d) 0.006
Answer
C
Question. The radius of germanium (Ge) nuclide is measured to be twice the radius of 49Be. The number of nucleons in Ge are
(a) 72
(b) 73
(c) 74
(d) 75
Answer
A
Question. The volume occupied by an atom is greater than the volume of the nucleus by a factor of about
(a) 101
(b) 105
(c) 1010
(d) 1015
Answer
D
Question. The energy required to break one bond in DNA is 10–20 J. This value in eV is nearly
(a) 6
(b) 0.6
(c) 0.06
(d) 0.006
Answer
C
Question. The energy equivalent of 0.5 g of a substance is
(a) 4.5 × 1016 J
(b) 4.5 × 1013 J
(c) 1.5 × 1013 J
(d) 0.5 × 1013 J
Answer
B
Question. The mass density of a nucleus varies with mass number A as
(a) A2
(b) A
(c) constant
(d) 1/A
Answer
C
Question. The ratio of the radii of the nuclei 13Al27 and 52Te125 is approximately
(a) 6 : 10
(b) 13 : 52
(c) 40 : 177
(d) 14 : 73
Answer
A
Question. How does the Binding Energy per nucleon vary with the increase in the number of nucleons?
(a) Decrease continuously with mass number.
(b) First decreases and then increases with increase in mass number.
(c) First increases and then decreases with increase in mass number.
(d) Increases continuously with mass number.
Answer
C
Question. A nucleus AZX has mass represented by M(A, Z). If Mp and Mn denote the mass of proton and neutron respectively and B.E. the binding energy in MeV, then
(a) B.E. = [ZMp + (A – Z)Mn – M(A, Z)]c2
(b) B.E. = [ZMp + AMn – M(A, Z)]c2
(c) B.E. = M(A, Z) – ZMp – (A – Z)Mn
(d) B.E. = [M(A, Z) – ZMp – (A – Z)Mn]c2
Answer
A
Question. The mass of a 37Li nucleus is 0.042 u less than the sum of the masses of all its nucleons. The binding energy per nucleon of 37Li nucleus is nearly
(a) 46 MeV
(b) 5.6 MeV
(c) 3.9 MeV
(d) 23 MeV
Answer
B
Question. Which of the following are suitable for the fusion process?
(a) Light nuclei
(b) Heavy nuclei
(c) Element lying in the middle of the periodic table
(d) Middle elements, which are lying on binding energy curve.
Answer
A
Question. Mn and Mp represent the mass of neutron and proton respectively. An element having mass M has N neutrons and Z protons, then the correct relation will be
(a) M < {N · Mn + Z · Mp}
(b) M > {N · Mn + Z · Mp}
(c) M = {N · Mn + Z · Mp}
(d) M = N {Mn + Mp}
Answer
A
Question. If M(A; Z), Mp and Mn denote the masses of the nucleus AZX, proton and neutron respectively in units of u (1 u = 931.5 MeV/c2) and BE represents its binding energy in MeV, then
(a) M(A, Z) = ZMp + (A – Z)Mn – BE
(b) M(A, Z) = ZMp + (A – Z)Mn + BE/c2
(c) M(A, Z) = ZMp + (A – Z)Mn – BE/c2
(d) M(A, Z) = ZMp + (A – Z)Mn + BE
Answer
C
Question. Fission of nuclei is possible because the binding energy per nucleon in them
(a) increases with mass number at low mass numbers
(b) decreases with mass number at low mass numbers
(c) increases with mass number at high mass numbers
(d) decreases with mass number at high mass numbers.
Answer
D
Question. The mass of proton is 1.0073 u and that of neutron is 1.0087 u (u = atomic mass unit). The binding energy of 24He is
(Given helium nucleus mass ≈ 4.0015 u)
(a) 0.0305 J
(b) 0.0305 erg
(c) 28.4 MeV
(d) 0.061 u
Answer
C
Question. Energy released in nuclear fission is due to
(a) some mass is converted into energy
(b) total binding energy of fragments is more than the binding energy of parental element
(c) total binding energy of fragments is less than the binding energy of parental element
(d) total binding energy of fragments is equal to the binding energy of parental element.
Answer
A
Question. The binding energy per nucleon is maximum in case of
(a) 24He
(b) 2656Fe
(c) 14156Ba
(d) 92235U
Answer
B
Question. Which of the following statements is true for nuclear forces?
(a) They obey the inverse square law of distance.
(b) They obey the inverse third power law of distance.
(c) They are short range forces.
(d) They are equal in strength to electromagnetic forces.
Answer
C
Question. a-particle consists of
(a) 2 protons only
(b) 2 protons and 2 neutrons only
(c) 2 electrons, 2 protons and 2 neutrons
(d) 2 electrons and 4 protons only
Answer
B
Question. The energy equivalent of one atomic mass unit is
(a) 1.6 × 10–19 J
(b) 6.02 × 1023 J
(c) 931 MeV
(d) 9.31 MeV
Answer
C
Question. The average binding energy of a nucleon inside an atomic nucleus is about
(a) 8 MeV
(b) 8 eV
(c) 8 J
(d) 8 erg
Answer
A
Question. If the nuclear force between two protons, two neutrons and between proton and neutron is denoted by Fpp, Fnn and Fpn respectively, then
(a) Fpp ≈ Fnn ≈ Fpn
(b) Fpp ≠ Fnn and Fpp = Fnn
(c) Fpp = Fnn = Fpn
(d) Fpp ≠ Fnn ≠ Fpn
Answer
C
Question. The rate of radioactive disintegration at an instant for a radioactive sample of half life 2.2 × 109 s is 1010 s–1. The number of radioactive atoms in the sample at that instant is,
(a) 3.17 × 1020
(b) 3.17 × 1017
(c) 3.17 × 1018
(d) 3.17 × 1019
Answer
D
Question. A nucleus ruptures into two nuclear parts, which have their velocity ratio equal to 2 : 1. What will be the ratio of their nuclear size (nuclear radius)?
(a) 31/2 : 1
(b) 1 : 31/2
(c) 21/3 : 1
(d) 1 : 21/3
Answer
D
Question. The energy equivalent of 0.5 g of a substance is
(a) 4.5 × 1016 J
(b) 4.5 × 1013 J
(c) 1.5 × 1013 J
(d) 0.5 × 1013 J
Answer
B
Question. A nucleus of uranium decays at rest into nuclei of thorium and helium. Then
(a) The helium nucleus has more momentum than the thorium nucleus.
(b) The helium nucleus has less kinetic energy than the thorium nucleus.
(c) The helium nucleus has more kinetic energy than the thorium nucleus.
(d) The helium nucleus has less momentum than the thorium nucleus.
Answer
C
Question. The binding energy per nucleon of 37Li and 24He nuclei are 5.60 MeV and 7.06 MeV respectively. In the nuclear reaction 37Li + 11H → 24He + 24He +Q the value of energy Q released is
(a) 19.6 MeV
(b) –2.4 MeV
(c) 8.4 MeV
(d) 17.3 MeV
Answer
D
Question. For a radioactive material, half-life is 10 minutes. If initially there are 600 number of nuclei, the time taken (in minutes) for the disintegration of 450 nuclei is
(a) 20
(b) 10
(c) 30
(d) 15
Answer
A
Question. Radioactive material ‘A’ has decay constant ‘8 l’ and material ‘B’ has decay constant ‘l’. Initially they have same number of nuclei. After what time, the ratio of number of nuclei of material ‘B’ to that ‘A’ will be (1/e)?
(a) 1/7λ
(b) 1/8λ
(c) 1/9λ
(d) 1/λ
Answer
B
Question. The half-life of a radioactive substance is 30 minutes. The time (in minutes) taken between 40% decay and 85% decay of the same radioactive substance is
(a) 15
(b) 30
(c) 45
(d) 60
Answer
D
Question. A radioisotope X with a half life 1.4 × 109 years decays to Y which is stable. A sample of the rock from a cave was found to contain X and Y in the ratio 1 : 7. The age of the rock is
(a) 1.96 × 109 years
(b) 3.92 × 109 years
(c) 4.20 × 109 years
(d) 8.40 × 109 years
Answer
C
Question. The half life of a radioactive nucleus is 50 days. The time interval (t2 – t1) between the time t2 when 2/3 of it has decayed and the time t1 when 1/3 of it had decayed is
(a) 30 days
(b) 50 days
(c) 60 days
(d) 15 days
Answer
B
Question. The half life of a radioactive isotope X is 50 years. It decays to another element Y which is stable. The two elements X and Y were found to be in the ratio of 1 : 15 in a sample of a given rock. The age of the rock was estimated to be
(a) 150 years
(b) 200 years
(c) 250 years
(d) 100 years
Answer
B
Question. The half life of a radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. The two elements ‘X’ and ‘Y’ were found to be in the ratio 1 : 7 in a sample of a given rock. The age of the rock is estimated to be
(a) 80 years
(b) 100 years
(c) 40 years
(d) 60 years
Answer
D
Question. a-particles, b-particles and g-rays are all having same energy. Their penetrating power in a given medium in increasing order will be
(a) Υ, a, b
(b) a, b, Υ
(c) b, a, Υ
(d) b, Υ, a
Answer
B
Question. A mixture consists of two radioactive materials A1 and A2 with half lives of 20 s and 10 s respectively. Initially the mixture has 40 g of A1 and 160 g of A2. The amount of the two in the mixture will become equal after
(a) 60 s
(b) 80 s
(c) 20 s
(d) 40 s
Answer
D
Question. A radioactive nucleus of mass M emits a photon of frequency u and the nucleus recoils. The recoil energy will be
(a) Mc2 – hu
(b) h2u2/2Mc2
(c) zero
(d) hu
Answer
B
Question. The decay constant of a radio isotope is λ. If A1 and A2 are its activities at times t1 and t2 respectively, the number of nuclei which have decayed during the time (t1 – t2)
(a) A1t1 – A2t2
(b) A1 – A2
(c) (A1 – A2)/λ
(d) λ(A1 – A2)
Answer
C
Question. In the nuclear decay given below

the particles emitted in the sequence are
(a) y, b, a
(b) b, y, a
(c) a, b, y
(d) b, a, y
Answer
D
Question. A nucleus nmX emits one a particle and two β–particles. The resulting nucleus is

Answer
C
Question. Two radioactive nuclei P and Q, in a given sample decay into a stable nucleus R. At time t = 0, number of P species are 4 N0 and that of Q are N0 . Half-life of P (for conversion to R) is 1 minute where as that of Q is 2 minutes. Initially there are no nuclei of R present in the sample. When number of nuclei of P and Q are equal, the number of nuclei of R present in the sample would be
(a) 2 N0
(b) 3 N0
(c) 9N0/2
(d) 5N0/2
Answer
C
Question. The number of beta particles emitted by a radioactive substance is twice the number of alpha particles emitted by it. The resulting daughter is an
(a) isomer of parent
(b) isotone of parent
(c) isotope of parent
(d) isobar of parent
Answer
C
Question. Two radioactive materials X1 and X2 have decay constants 5λ and λ respectively. If initially they have the same number of nuclei, then the ratio of the number of nuclei of X1 to that X2 will be 1/e after a time
(a) 1/4λ
(b) e/λ
(c) λ
(d) 1/2λ
Answer
A
Question. The half life of radium is about 1600 years. If 100 g of radium existing now, 25 g will remain unchanged after
(a) 4800 years
(b) 6400 years
(c) 2400 years
(d) 3200 years
Answer
D
Question. In a radioactive decay process, the negatively charged emitted b-particles are
(a) the electrons produced as a result of the decay of neutrons inside the nucleus
(b) the electrons produced as a result of collisions between atoms
(c) the electrons orbiting around the nucleus
(d) the electrons present inside the nucleus.
Answer
A
Question. The activity of a radioactive sample is measured as N0 counts per minute at t = 0 and N0/e counts per minute at t = 5 minutes. The time (in minutes) at which the activity reduces to half its value is

Answer
D
Question. A sample of radioactive element has a mass of 10 g at an instant t = 0. The approximate mass of this element in the sample after two mean lives is
(a) 1.35 g
(b) 2.50 g
(c) 3.70 g
(d) 6.30 g
Answer
A
Question. Two radioactive substances A and B have decay constants 5l and l respectively. At t = 0 they have the same number of nuclei. The ratio of number of nuclei of A to those of B will be (1/e)2 after a time interval
(a) 4λ
(b) 2λ
(c) 1/2λ
(d) 1/4λ
Answer
A
Question. In a radioactive material the activity at time t1 is R1 and at a later time t2, it is R2. If the decay constant of the material is λ, then
(a) R1 = R2
(b) R1 R2e -λ(t1− t2)
(c) R1 R2eλ(t1 – t2)
(d) R1 = R2(t2/t1)
Answer
B
Question. Which rays contain (positive) charged particles?
(a) α-rays
(b) β-rays
(c) y-rays
(d) X-rays
Answer
A
Question. In the reaction 12H +31H → 42He + 10n, if the binding energies of 12H, 31H and 24He are respectively a, b and c (in MeV), then the energy (in MeV) released in this reaction is
(a) a + b + c
(b) a + b – c
(c) c – a – b
(d) c + a – b
Answer
C
Question. A sample of radioactive element containing 4 × 1016 active nuclei. Half life of element is 10 days, then number of decayed nuclei after 30 days
(a) 0.5 × 1016
(b) 2 × 1016
(c) 3.5 × 1016
(d) 1 × 1016
Answer
C
Question. Half life of a radioactive element is 12.5 hours and its quantity is 256 g. After how much time its quantity will remain1 g?
(a) 50 hrs
(b) 100 hrs
(c) 150 hrs
(d) 200 hrs
Answer
B
Question. A deutron is bombarded on 8O16 nucleus then a-particle is emitted. The product nucleus is
(a) 7N13
(b) 5B10
(c) 4Be9
(d) 7N14
Answer
D
Question. X(n, a) 37Li, then X will be
(a) 510B
(b) 59B
(c) 411Be
(d) 24He
Answer
A
Question. Alpha particles are
(a) neutrally charged
(b) positron
(c) protons
(d) ionized helium atoms
Answer
D
Question. For the given reaction, the particle X is 6C11 → 5B11 + b+ + X
(a) neutron
(b) anti neutrino
(c) neutrino
(d) proton
Answer
C
Question. The relation between l and T1/2 as (T1/2 → half life)
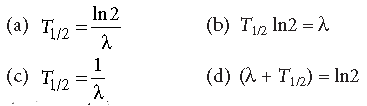
Answer
A
Question. After 1a and 2β-emissions
(a) mass number reduces by 6
(b) mass number reduces by 4
(c) mass number reduces by 2
(d) atomic number remains unchanged
Answer
B, D



