VBQs Chemical Kinetics Class 12 Chemistry with Chemical Kinetics has been provided below for standard students. We have provided chapter wise VBQ for Class 12 Chemistry with Chemical Kinetics. The following Chemical Kinetics Class 12 Chemistry value based questions with answers will come in your exams. Students should understand the concepts and learn the solved cased based VBQs provided below. This will help you to get better marks in class 12 examinations.
Chemical Kinetics VBQs Class 12 Chemistry
Question. The rate constant of a first order reaction is 15 × 10–3 s–1. How long will 5.0 g of this reactant take to reduce to 3.0 g?
(a) 34.07 s
(b) 7.57 s
(c) 10.10 s
(d) 15 s
Answer
A
Question. Which of the following statements for order of reaction is not correct?
(a) Order can be determined experimentally.
(b) Order of reaction is equal to the sum of powers of concentration terms in rate law expression.
(c) Order cannot be fractional.
(d) Order is not affected by stoichiometric coefficient of the reactants.
Answer
C
Question. The rate law for a reaction,
A + B → C + D is given by the expression k[A].
The rate of reaction will be
(a) doubled on doubling the concentration of B
(b) halved on reducing the concentration of A to half
(c) decreased on increasing the temperature of the reaction
(d) unaffected by any change in concentration or temperature.
Answer
B
Question. The half-life of the reaction X → Y, following first order kinetics, when the initial concentration of A is 0.01 mol L–1 and initial rate is 0.00352 mol L–1 min–1 will be
(a) 19.69 min
(b) 1.969 min
(c) 7.75 min
(d) 77.5 min
Answer
B
Question. When a chemical reaction takes place,during the course of the reaction the rate of reaction
(a) keeps on increasing with time
(b) remains constant with time
(c) keeps on decreasing with time
(d) shows irregular trend with time.
Answer
C
Question. The reaction 2X → Y + Z would be zero order reaction when
(a) rate remains unchanged at any concentration of Y and Z
(b) rate of reaction doubles if concentration of Y is doubled.
(c) rate of reaction remains same at any concentration of X
(d) rate of reaction is directly proportional to square of concentration of X.
Answer
C
Question. Which of the following statements is not correct?
(a) For a zero order reaction, t1/2 is proportional to initial concentration.
(b) For a reaction
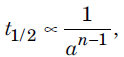
where n is order of the reaction.
(c) The unit of rate constant for a reaction is mol1 – n L n – 1 s–1 where n is order of the reaction.
(d) The unit of rate of reaction changes with order of reaction.
Answer
D
Question. The decomposition of dimethyl ether is a fractional order reaction. The rate of reaction is given by rate = k(pCH3COCH3)3/2. If the pressure is measured in bar and time in minutes, then what are the units of rate and rate constant respectively?
(a) bar min–1, bar2 min–1
(b) bar min–1, bar–1/2 min–1
(c) bar–1/2 min–1, bar2 min–1
(d) bar min–1, bar1/2 min–1
Answer
B
Question. Consider the reaction, 2N2O5 → 4NO2 + O2.In the reaction NO2 is being formed at the rate of 0.0125 mol L–1 s–1. What is the rate of reaction at this time?
(a) 0.0018 mol L–1 s–1
(b) 0.0031 mol L–1 s–1
(c) 0.0041 mol L–1 s–1
(d) 0.050 mol L–1 s–1
Answer
B
Question. For a reaction R → P, the concentration of a reactant changes from 0.05 M to 0.04 M in 30 minutes. What will be the average rate of reaction in minutes?
(a) 4 × 10–4 M min–1
(b) 8 × 10–4 M min–1
(c) 3.3 ×10–4 M min–1
(d) 2.2 × 10–4 M min–1
Answer
C
Question. For the reaction 4NH3 + 5O2 → 4NO + 6H2O,if the rate of disappearance of NH3 is 3.6 × 10–3 mol L–1 s–1, what is the rate of formation of H2O?
(a) 5.4 × 10–3 mol L–1 s–1
(b) 3.6 × 10–3 mol L–1 s–1
(c) 4 × 10–4 mol L–1 s–1
(d) 0.6 × 10–4 mol L–1 s–1
Answer
A
Question. The number of molecules of the reactants taking part in a single step of the reaction is indicative of
(a) order of a reaction
(b) molecularity of a reaction
(c) fast step of the mechanism of a reaction
(d) half-life of the reaction.
Answer
B
Question. The rate of a gaseous reaction is given by the expression k[A]2[B]3. The volume of the reaction vessel is reduced to one half of the initial volume.
What will be the reaction rate as compared to the original rate a?
(a) 1/8 a
(b) 1/2 a
(c) 2a
(d) 32a
Answer
D
Question. For a reversible reaction, A + B ⇌ C + D, the graph for rate of reaction with time is given below.
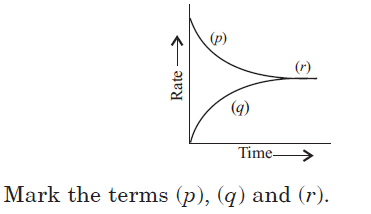
(a) (p) – rate of backward reaction, (q) – rate of forward reaction, (r) – equilibrium
(b) (p) – rate of forward reaction, (q) – rate of backward reaction, (r) – equilibrium
(c) (p) – concentration of products, (q) – concentration of reactants, (r) – rate of reaction
(d) (p) – instantaneous rate of reaction, (q) – variation of rate, (r) – average rate of reaction
Answer
B
Question. The decomposition of a substance follows first order kinetics. If its concentration is reduced to 1/8 of its initial value in 12 minutes, the rate constant of the decomposition system is
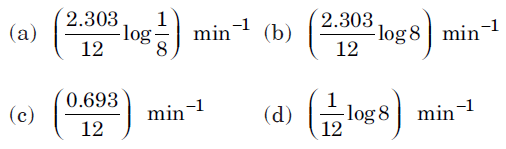
Answer
B
Question. In a reaction 2HI → H2 + I2, the concentration of HI decreases from 0.5 mol L–1 to 0.4 mol L–1 in 10 minutes. What is the rate of reaction during this interval?
(a) 5 × 10–3 M min–1
(b) 2.5 × 10–3 M min–1
(c) 5 × 10–2 M min–1
(d) 2.5 × 10–2 M min–1
Answer
A
Question. In pseudo unimolecular reactions,
(a) both the reactants are present in low concentration
(b) both the reactants are present in same concentration
(c) one of the reactants is present in excess
(d) one of the reactants is non-reactive
Answer
C
Question. For a unimolecular reaction,
(a) the order and molecularity of the slowest step are equal to one
(b) molecularity of the reaction can be zero, one or two
(c) more than one reacting species are involved in one step
(d) molecularity of the reaction can be determined only experimentally.
Answer
A
Question. For a general reaction X Y, the plot of conc. of X vs time is given in the figure. What is the order of the reaction and what are the units of rate constant?
(a) Zero, mol L–1 s–1
(b) First, mol L–1 s–1
(c) First, s–1
(d) Zero, L mol–1 s–1
Answer
A
Question. Radioactive disintegration is an example of
(a) zero order reaction
(b) first order reaction
(c) second order reaction
(d) third order reaction.
Answer
B
Question. Which of the following is an example of a fractional order reaction?
(a) NH4NO2 → N2 + 2H2O
(b) NO + O3 → NO2 + O2
(c) 2NO + Br2 → 2NOBr
(d) CH3CHO → CH4 + CO
Answer
D
Question. The chemical reaction, 2O3 → 3O2 proceeds as
O3 ⇌ O2 + [O] (fast)
[O] + O3 → 2O2 (slow)
The rate law expression will be
(a) Rate = k[O][O3]
(b) Rate = k[O3]2 [O2]–1
(c) Rate = k[O3]2
(d) Rate = k[O2][O]
Answer
B
Question. The value of rate of a pseudo first order reaction depends upon
(a) the concentration of both the reactants present in the reaction
(b) the concentration of the reactant present in small amount
(c) the concentration of the reactant present in excess
(d) the value of DH of the reaction.
Answer
B
Question. Nitrogen dioxide (NO2) dissociates into nitric oxide (NO) and oxygen (O2) as follows:
2NO2 → 2NO + O2
If the rate of decrease of concentration of NO2 is 6.0 × 10–12 mol L–1 s–1, what will be the rate of increase of concentration of O2?
(a) 3 × 10–12 mol L–1 s–1
(b) 6 × 10–12 mol L–1 s–1
(c) 1 × 10–12 mol L–1 s–1
(d) 1.5 × 10–12 mol L–1 s–1
Answer
A
Question. For a reaction P + Q → 2R + S. Which of the following statements is incorrect?
(a) Rate of disappearance of P = Rate of appearance of S
(b) Rate of disappearance of Q = 2 × Rate of appearance of R
(c) Rate of disappearance of P = Rate of disappearance of Q
(d) Rate of disappearance of Q = 1/2 × Rate of appearance of R
Answer
B
Question. The rate constant for the reaction,
2N2O5 → 4NO2 + O2 is 2 × 10–5 s–1. If rate of reaction is 1.4 × 10–5 mol L–1 s–1, what will be the concentration of N2O5 in mol L–1?
(a) 0.8
(b) 0.7
(c) 1.2
(d) 1
Answer
B
Question. Consider the reaction P → Q. The concentration of both the reactants and the products varies exponentially with time. Which of the following figures correctly describes the change in concentration of reactants and products with time?
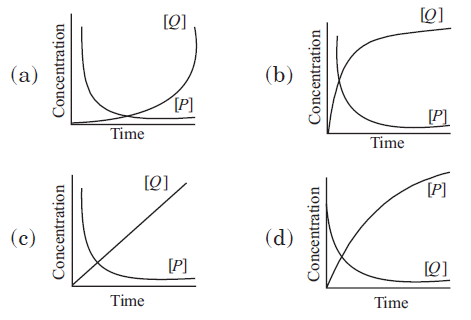
Answer
B
Question. For the reaction, 2N2O5 → 4NO2 + O2,the rate of reaction can be expressed in terms of time and concentration by the expression:
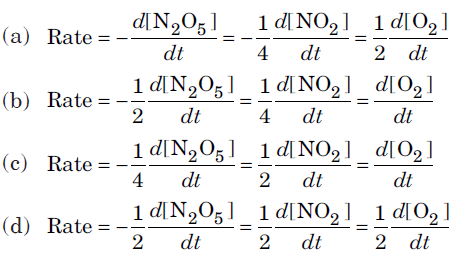
Answer
B
Question. In a reaction 2X → Y, the concentration of X decreases from 3.0 moles/litre to 2.0 moles/litre in 5 minutes. The rate of reaction is
(a) 0.1 mol L–1 min–1
(b) 5 mol L–1 min–1
(c) 1 mol L–1 min–1
(d) 0.5 mol L–1 min–1
Answer
A
Question. The unit of rate and rate constant are same for a
(a) zero order reaction
(b) first order reaction
(c) second order reaction
(d) third order reaction.
Answer
A
SHORT ANSWER TYPE QUESTIONS
Question. The rate of a particular reaction quadruples when the temperature changes from 293 K to 313 K. Calculate activation energy.
Answer.
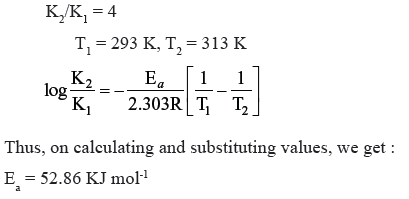
Question. If the decomposition of nitrogen oxide as
2N2O5 → 4NO2 + O2
follows a first order kinetics.
(a) Calculate the rate constant for a 0.05M solution if the instantaneous rate is 1.5 × 10−6 mol/l/s ?
Answer.
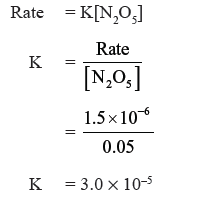
(b) What concentration of N2O5 would give a rate of 2.45 × 10−5 mol L-1 s-1 ?
Answer.
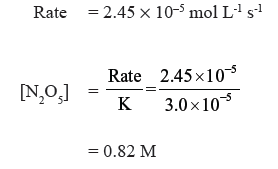
Question. Write the difference between order and molecularity of reaction.
Answer.
Order Molecularity
1. It is the sum of the powers of concentration 1. It is the number of reacting species undergoing
terms in the rate law expression. simultaneously collision in a reaction.
2. It is determined experimentally. 2. It is a theoretical concept.
3. Order of reaction need not to be a whole num 3. It is whole number only.
ber.
4. Order of reaction can be zero. 4. It can’t be zero or fractional.
Question. Consider the decomposition reaction :
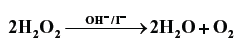
This reaction takes place in two steps as given below :
Step 1. H2O2 + I− → H2O + IO− (slow)
Step 2. H2O2 + IO− → H2O + I− + O2 (fast)
(a) Determine rate law expression.
(b) Determine the order of reaction.
Answer. (a) Rate = K[H2O2][I–] because second step is rate determining step.
(b) Order = 1 + 1 = 2
Question. The decomposition of hydrocarbon follows the equation K = (4.5 × 1011 s-1) e-28000k/T. Calculate Ea.
Answer.

Question. A reaction is of second order with respect to a reactant. How is the rate of reaction affected if the conc. of the reactant is reduced to half. What is the unit of rate constant for such a reaction ?
Answer.
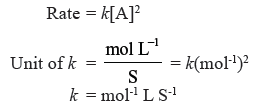
Question. For a first order reaction time taken for half of the reaction to complete is t1 and ¾ of the reaction to complete is t2. How are t1 and t2 related ?
Answer. t2 = 2t1 because for 3/4th of the reaction to complete time required is equal to two half lives.
Question.
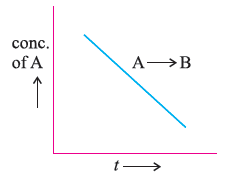
(a) What is the order of the reaction ?
(b) What is the slope of the curve ?
Answer. (a) Zero order reaction.
(b) [R] = [R0] – kt ∴ Slope = – k
Question. Derive an expression to calculate time required for completion of zero order reaction.
Answer. For a zero order reaction,
R = [R]0 – kt
For completion of the reaction [R] =0
∴ kt = [R]0
Or t =[R]0 /k
Question. For the reaction N2 (g) + 3H2 (g) → 2NH3 (g)
How is the rate of formation of ammonia related to the rate of disappearance of H2 ?
Answer.
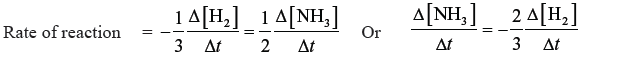
Question. The rate of a gaseous reaction becomes half when volume of the vessel is doubled. What is the order of reaction ?
Answer. Suppose, order of reaction is n and the reaction is A (g) → Products
Rate = k[A]n …(i)
When volume is doubled, molar conc. becomes half and rate of reaction gets halved.
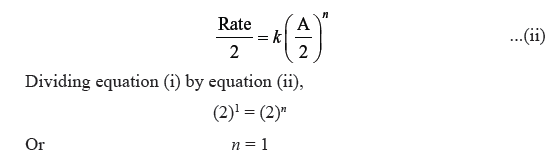
Question. A reaction which is first order with respect to A has rate constant 6 min-1. If we start with [A] = 0.5 mol L-1, when would [A] reach the value of 0.05 ML-1 ?
Answer.
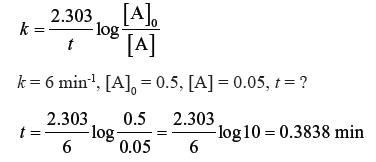
Question. The conversion of the molecules X to Y follows second order kinetics. If the concentration of X is increased to three times, how will it affect the rate of formation of Y ?
Answer. 9 times
Question. A first order reaction has a rate constant 1.15 × 10-3 s-1. How long will 5 gram of this reactant take to reduce to 3 grams ?
Answer. t = 444 seconds
Question. 4NH3 + 5O2 → 4NO + 6H2O. If rate of formation of NO is 6 × 10−4 atm min-1, calculate the rate of formation of H2O.
Answer. 9.0 × 10−4 atm min-1
Question. Consider a certain reaction A → Product with K = 2.0 × 10-2 s-1. Calculate the concentration of A remaining after 100 s, if the initial concentration of A is 1.0 mol L-1.
Answer. [A] = 0.135 M
Question. The half life period of a first order reaction is 60 min. What % will be left after 240 mins. ?
Answer. 6.25%
Question. Time for half change for a first order reaction is 25 min. What time will be required for 99% reaction ?
Answer. 166.16 mins.