VBQs Electrochemistry Class 12 Chemistry with Electrochemistry has been provided below for standard students. We have provided chapter wise VBQ for Class 12 Chemistry with Electrochemistry. The following Electrochemistry Class 12 Chemistry value based questions with answers will come in your exams. Students should understand the concepts and learn the solved cased based VBQs provided below. This will help you to get better marks in class 12 examinations.
Electrochemistry VBQs Class 12 Chemistry
VERY SHORT ANSWER TYPE QUESTIONS
Question. Why is it not possible to measure single electrode potential ?
Answer. Because the half cell containing single electrode cannot exist independently, as charge cannot flow on its own in a single electrode.
Question. Name the factor on which emf of a cell depends.
Answer. Emf of a cell depends on following factors :
(a) Nature of reactants
(b) Concentration of solution in two half cells
(c) Temperature
Question. What is the effect of temperature on the electrical conductance of metal ?
Answer. Temperature increases, electrical conductance decreases.
Question. What is the effect of temperature on the electrical conductance of electrolyte ?
Answer. Temperature increases, electrical conductance increases.
Question. What is the relation between conductance and conductivity ?
Answer.
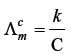
Question. What is the electrolyte used in a dry cell ?
Answer. A paste of NH4Cl.
SHORT ANSWER-I TYPE QUESTIONS
Question. How can you increase the reduction potential of an electrode for the reaction :
Mn+ (aq) + ne– → M (s)
Answer. Nernst equation is :
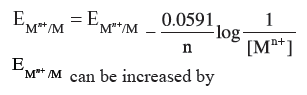
(a) Increase in concentration of Mn+ ions in solution.
(b) By increasing the temperature.
Question. Calculate emf of the following cell at 298 K :
Mg (s) + 2Ag+ (0.0001M) → Mg2+ (0.130 M) + 2Ag (s)
[Given : Eθcell = 3.17 V]
Answer. n = 2
The Nernst equation for the cell is :
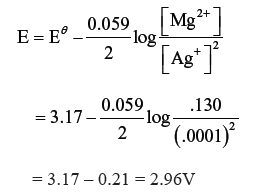
Question. Suggest a way to determine the Λmº value of water.
Answer.
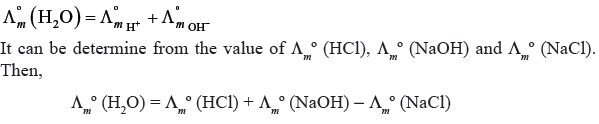
Question. How much electricity in term of Faraday is required to produce 40 gram of Al from Al2O3 ? (Atomic mass of Al = 27 g/mol)
Answer.Al3+ + 3e– → Al
27 gram of Al require electricity = 3F
40 gram of Al require electricity = 3F/27× 40 = 4.44 F
Question. Predict the product of electrolysis of an aqueous solution of CuCl2 with an inert electrode.
Answer. CuCl2 (s) + Aq → Cu2+ + 2Cl–
H2O → H+ + OH–
At cathode (Reduction) : Cu2+ will be reduced in preference to H+ ions.
Cu2+ + 2e– → Cu(s)
At anode (Oxidation) : Cl– ions will be oxidized in preference to OH– ions.
Cl– → ½Cl2 + 1e–
Thus, Cu will be deposited at cathode and Cl2 will be liberated at anode.
Question. Calculate Λºm for CaCl2 and MgSO4 from the following data :
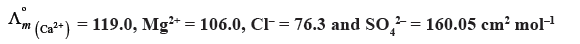
Answer.
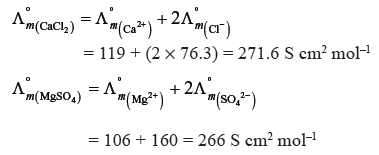
Question. Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Answer. H+ + e– → ½H2 n = 1
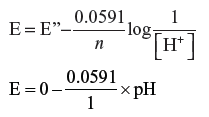
E = − 0.0591 × 10 v
E = – 0.591/V
Question. If a current of 0.5 amp flows through a metallic wire for 2 hours, how many electrons would flow through the wire ?
Answer. q = i × t = 0.5 × 2 × 60 × 60 = 3600 C
96500 Coulombs are equal to 6.022 × 1023e–
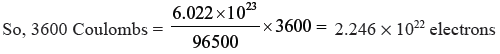
Question. How much electricity is required in Coulomb for the oxidation of 1 mole of FeO to Fe2O3 ?
Answer. Fe2+ → Fe3+ + 1e–
So, 1F = 1 × 96500 C = 96500 C
Question. The conductivity of a 0.20M solution of KCl at 298K is 0.0248 S cm–1.
Calculate molar conductivity.
Answer.
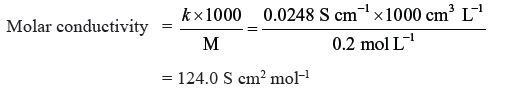
Question. Explain Kohlrausch’s law of independent migration of ions.
Answer. It states that at infinite dilution, molar conductivity of an electrolyte is equal to sum of contributions due to cation as well as anion.

Question. The standard reduction potential for the Zn2+ (aq)/Zn (s) half cell is – 0.76V.
Write the reactions occurring at the electrodes when coupled with standard hydrogen electrode (SHE).
Answer. At anode : Zn (s) → Zn2+ (aq) + 2e–
At cathode : 2H+ + 2e− → H2 (g)
Zn (s) + 2H+ (al) → Zn2+ (aq) + H2 (g)
Question. Calculate the electrode potential of a copper wire dipped in 0.1M CuSO4 solution at 25ºC. The standard electrode potential of copper is 0.34 Volt.
Answer. The electrode reaction written as reduction potential is
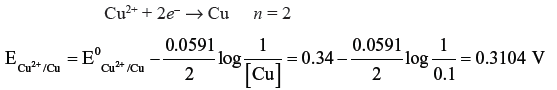
Question. Two metals A and B have reduction potential values – 0.76 V and + 0.34 V respectively. Which of these will liberate H2 from dil. H2SO4 ?
Answer. Metal having higher oxidation potential will liberate H2 from H2SO4. Thus, A will liberate H2 from H2SO4.
Question. How does conc. of sulphuric acid change in lead storage battery when current is drawn from it ?
Answer. Concentration of sulphuric acid decreases.
Question. What type of a battery is lead storage cell ? Write the anode and cathode reaction and overall reaction occurring in a lead storage battery during discharging and recharging of cell.
Answer. It is a secondary cell.
Anode reaction : Pb + SO42− → PbSO4 + 2e−
Cathode reaction : PbO2 + 4H+ + SO42− + 2e− → PbSO4 + 2H2O

Question. Why is alternating current used for measuring resistance of an electrolytic solution ?
Answer. The alternating current is used to prevent electrolysis so that the concentration of ions in the solution remains constant.
Question. Eθ values of MnO4−, Ce4+ and Cl2 are 1.507, 1.61 and 1.358 V respectively.Arrange these in order of increasing strength as oxidizing agent.
Answer. Cl2 < MnO4− < Ce4+
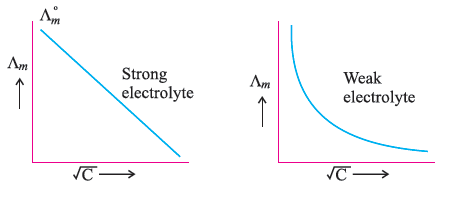
Question. Draw a graph between Λº m and √C for strong and weak electrolyte.
Answer.
Question. The conductivity of 0.02M solution of NaCl is 2.6 × 10-2 S cm-1. What is its molar conductivity ?
Answer.
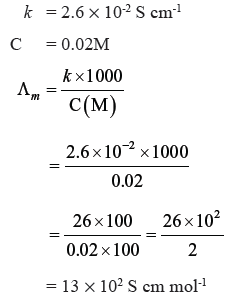
Question. Give products of electrolysis of an aqueous solution of AgNO3 with silver electrode.
Answer. At anode : Ag (s) → Ag+ + e–
At cathode : Ag+ + e− → Ag (s)
SHORT ANSWER-II TYPE QUESTIONS
Question. A solution of CuSO4 is electrolysed for 10 mins. with a current of 1.5 amperes.What is the mass of copper deposited at the cathode ?
Answer. I = 1.5 Ampere
Time = 10 × 60s = 600s
Q = I × t
= 1.5 × 600 = 900 C
Cu2+ + 2e− → Cu (s)
2F amount of electricity deposit copper = 63.5 g
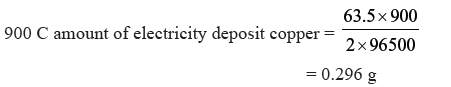
Question. Depict the galvanic cell in which the reaction
Zn (s) + 2Ag+ → Zn2+ + 2Ag (s)
takes place. Further show :
(a) Which of the electrode is negatively charged ?
(b) The carriers of the current in the cell.
(c) Individual reaction at each electrode.
Answer. Zn (s)|Zn2+ (aq) || Ag+ (aq)|Ag (s)
(a) Zn electrode (anode)
(b) Ions are carriers of the current in the cell.
(c) At anode :
Zn (s) → Zn2+ + 2e–
At cathode :
Ag+ + e− → Ag (s)
Question. The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10-3 S cm-1 ?
Answer. Cell constant = k × R
= 0.146 × 10-3 × 1500
= 0.219 cm-1
Question. Determine the values of equilibrium constant Kc and ΔGθ for the following reaction :
Ni (s) + 2Ag+ (aq) → Ni2+ (aq) + 2Ag (s) Eθ = 1.05 V
Answer. ΔGθ = − nFEθcell
n = 2, Eθcell = 1.05 V
F = 96500 C mol-1
ΔGθ = − 2 × 1.05 × 96500
= – 202.650 kJ
ΔGθ = − RT ln Kc
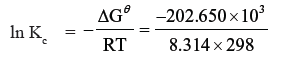
Kc = 3.32 × 1035
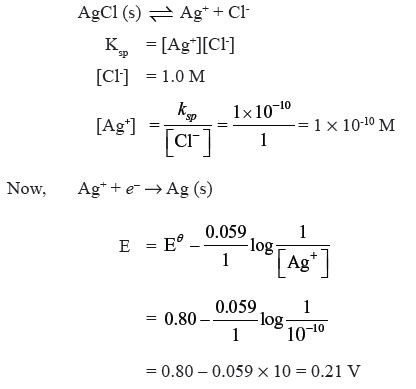
Question. The Ksp for AgCl at 298 K is 1.0 × 10-10. Calculate the electrode potential for Ag+/Ag electrode immersed in 1.0M KCl solution. Given EθAg+ /Ag = 0.80 V.
Answer.
Question. Estimate the minimum potential difference needed to reduce Al2O3 at 500ºC.
The free energy change for the decomposition reaction :
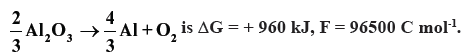
Answer.
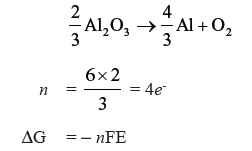
ΔG = 960 × 103 J, n = 4, F = 96500 C mol-1
960 × 103 = − 4 × 96500 × E
E = − 2.487 V
Minimum potential difference needed to reduce Al2O3 = – 2.487 V.
Question. Two electrolytic cells containing silver nitrate solution and copper sulphate solution are connected in series. A steady current of 2.5 amp was passed through them till 1.078 g of Ag were deposited. How long did the current flow ? What weight of copper will be deposited ? (Ag = 107.8 u, Cu = 63.5 u)
Answer. w = z × i × t
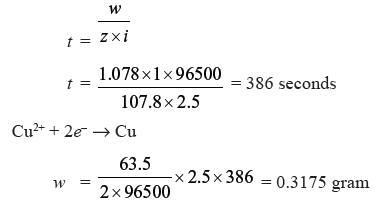
Question. A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5.0 amp for 20 minutes. What mass of the nickel will be deposited at the cathode ? (Ni = 58.7 u)
Answer.

Question. The cell in which the following reaction occurs :
2Fe3+ (aq) + 2I− (aq) → 2Fe2+ (aq) + I2 (s) has E0cell = 0.236 V.
Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Answer.
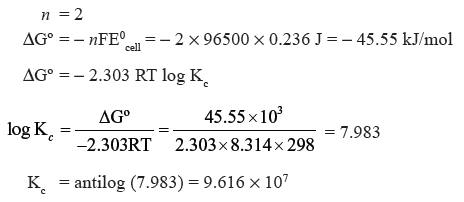
Question. The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1.
Calculate its degree of dissociation and dissociation constant. Given Λº (H+)
= 349.6 S cm2 mol-1, Λº (HCOO−) = 54.6 S cm2 mol-1.
Answer.
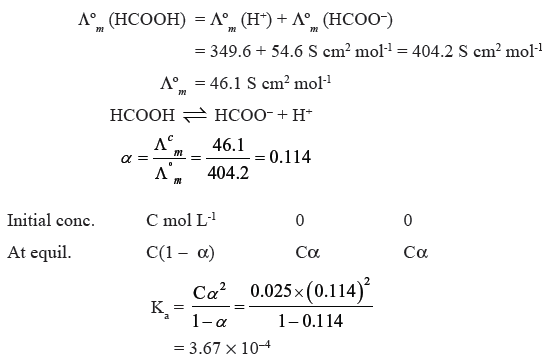
Question. Calculate the standard cell potentials of galvanic cells in which the following reaction take place :
2Cr (s) + 3Cd2+ (aq) → 2Cr3+ (aq) + 3Cd (s)
Also calculate ΔGº and equilibrium constant of the reaction.
Answer. E0cell = E0cathode − E0anode
= − 0.40 − (− 0.74) = 0.34 V
ΔGº = − nFE0cell = − 6 × 96500 × 0.34 = − 196860
= − 196860 J mol-1 = − 196.86 kJ/mol
− ΔGº = 2.303 RT log Kc
196860 = 2.303 × 8.314 × 298 log Kc
Or log Kc = 34.5014
Kc = antilog 34.5014 = 3.192 × 1034
Question. Calculate the potential of the following cell Sn4+ (1.5 M) + Zn → Sn2+ (0.5 M)+ Zn2+ (2M).
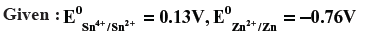
Will the cell potential ↑ or ↓ if the concentration of Sn4+ is increased ?
Answer.
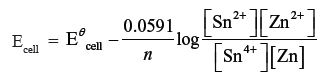
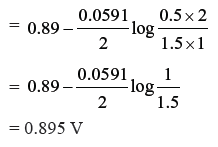
On increasing the concentration of Sn4+, EMF of the cell will increase.
Question. Eº (Cu2+/Cu) and Eº (Ag+/Ag) is + 0.337 V and + 0.799 V respectively. Make a cell whose EMF is +ve. If the concentration of Cu2+ is 0.01M and Ecell at 25ºC is zero, calculate the concentration of Ag+.
Answer. Cu is more reactive than silver, so that the cell is as Cu/Cu2+ (0.01M) || Ag+ (C)/Ag or cell reaction
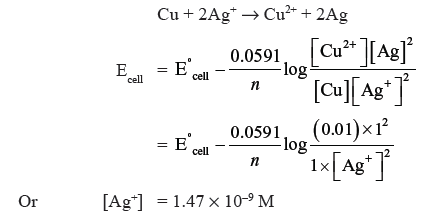
Question. Calculate the potential of the cell at 298 K :
Cd/Cd2+ (0.1M) || H+ (0.2M)/Pt, H2 (0.5 atm)
Given Eº for Cd2+/Cd = − 0.403 V, R = 8.314 J-1 mol-1, F = 96500 C mol-1.
Answer. The cell reaction is Cd + 2H+ (0.2M) → Cd2+ (0.1M) + H2 (0.5 atm)
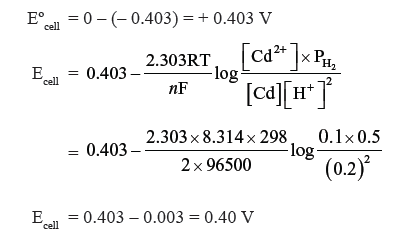
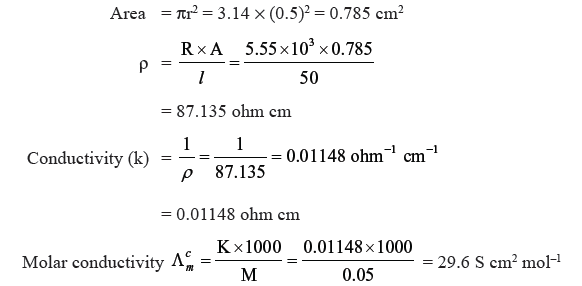
Question. The electrical resistance of a column of 0.05M NaOH solution of diameter 1 cm and length 50 cm is 5.55 × 103 ohm. Calculate its resistivity, conductivity and molar conductivity.
Answer. Diameter = 1 cm, radius = 0.5 cm
Question. Conductivity of saturated solution of BaSO4 at 315 K is 3.648 × 10–6 ohm–1cm–1 and that of water is 1.25 × 10–6 ohm–1 cm–1. Ionic conductance of Ba2+ and SO42– are 110 and 136.6 ohm–1 cm2 mol–1 respectively. Calculate the solubility of BaSO4 in g/L.
Answer.
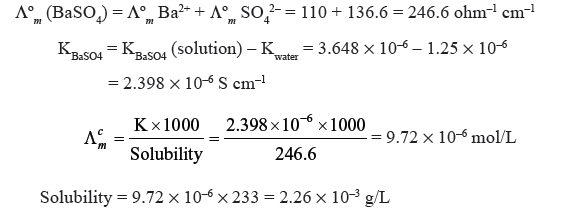
LONG ANSWER TYPE QUESTIONS (5 Marks)
Question. Conductivity of 0.00241M acetic acid is 7.896 × 10−5 S cm-1. Calculate its molar conductivity and if Λºm for acetic acid is 390.5 S cm2 mol-1, what is its dissociation constant ?
Answer.
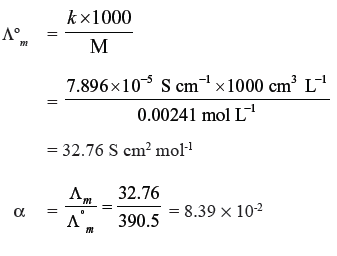
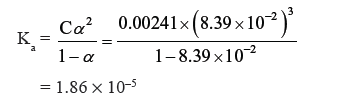
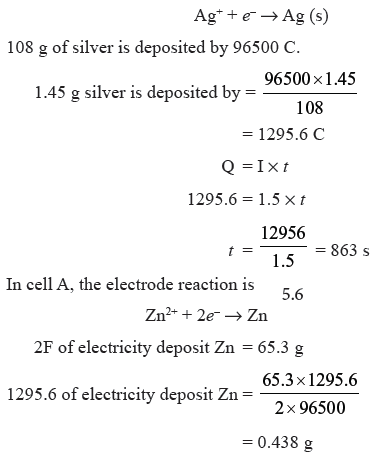
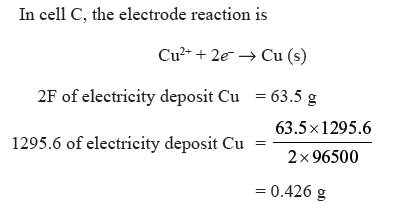
Question. Three electrolytic cells A, B, C containing solution of ZnSO4, AgNO3 and CuSO4 respectively all connected in series. A steady current of 1.5 amperes was passed through then until 1.45 g of silver deposited at the cathode of cell B. How long did the current flow ? What mass of copper and of zinc were deposited ?
Answer.
Question. (a) Define Kohlraush’s law.
(b) Suggest a way to determine the Λºm for CH3COOH.
(c) The Λºm for sodium acetate, HCl, NaCl are 91.0, 425.9 and 126.4 S cm2 mol-1 respectively at 298 K. Calculate Λºm for CH3COOH.
Answer. (a) The molar conductivity at a infinite dilution for a given salt can be expressed as the sum of the individual contribution from the ions of electrolyte.
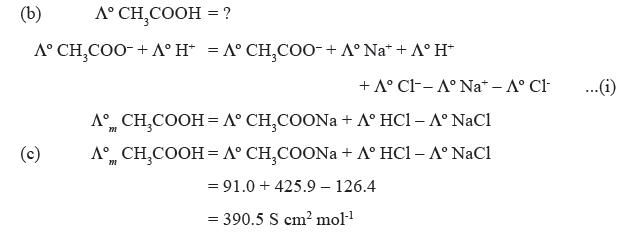
Question. (a) Define weak and strong electrolytes.
(b) The Eθ values corresponding to the following two reduction electrode processes are :
(i) Cu+/Cu = 0.52 V (ii) Cu2+/Cu+ = 0.16 V
Formulate the galvanic cell for their combination. Calculate the cell potential and ΔGº for the cell reaction.
Answer. (a) Weak electrolyte : The substance which partially ionized in solution is known as weak electrolyte. Example : NH4OH.
Strong electrolyte : The substance which completely ionized in solution is known as strong electrolyte. Example : NaCl.

Case Based MCQs
Case I : Read the passage given below and answer the following questions .
The study of the conductivity of electrolyte solutions is important for the development of electrochemical devices, for the characterisation of the dissociation equilibrium of weak electrolytes and for the fundamental understanding of charge transport by ions. The conductivity of electrolyte is measured for electrolyte solution with concentrations in the range of 10–3 to 10–1 mol L–1, as solution in this range of concentrations can be easily prepared. The molar conductivity (Λm) of strong electrolyte solutions can be nicely fit by Kohlrausch equation.
Λm = Λ°m – K√C …(i)
Where, Λ°m is the molar conductivity at infinite dilution and C is the concentration of the solution. K is an empirical proportionality constant to be obtained from the experiment. The molar conductivity of weak electrolytes, on the other hand, is dependent on the degree of dissociation of the electrolyte. At the limit of very dilute solution, the Ostwald dilution law is expected to be followed,

where, CA is the analytical concentration of the electrolyte and Kd is dissociation constant. The molar conductivity at infinite dilution can be decomposed into the contributions of each ion.

Where, l+ and l– are the ionic conductivities of positive and negative ions, respectively and n+ and n– are their stoichiometric coefficients in the salt molecular formula.
Question. If the molar conductivities at infinite dilution for NaI, CH3COONa and (CH3COO)2Mg are 12.69, 9.10 and 18.78 S cm2 mol–1 respectively at 25°C, then the molar conductivity of MgI2 at infinite dilution is
(a) 25.96 S cm2, mol–1
(b) 390.5 S cm2 mol–1
(c) 189.0 S cm2 mol–1
(d) 3.89 × 10–2 S cm2 mol–1
Answer
A
Question. Which of the following is a weak electrolyte in aqueous solution?
(a) K2SO4
(b) Na3PO4
(c) NaOH
(d) H2SO3
Answer
A
Question. Which statement about the term infinite dilution is correct?
(a) Infinite dilution refers to hypothetical situation when the ions are infinitely far apart.
(b) The molar conductivity at infinite dilution of NaCl can be measured directly in solution.
(c) Infinite dilution is applicable only to strong electrolytes.
(d) Infinite dilution refers to a real situation when the ions are infinitely far apart.
Answer
A
Question. Which of the following is the correct order of molar ionic conductivities of the following ions in aqueous solutions?
(a) Li+ < Na+ < K+ < Rb+
(b) Li+ > Na+ > K+ > Rb+
(c) Rb+ < Na+ < Li+ < K+
(d) Li+ < Rb+ < Na+ < K+
Answer
A
Question. Which of the following is a strong electrolyte in aqueous solution?
(a) HNO2
(b) HCN
(c) NH3
(d) HCl
Answer
D
Case II : Read the passage given below and answer the following questions.
The electrochemical cell shown below is concentration cell.
M | M2+ (saturated solution of a sparingly soluble salt, MX2) || M2+ (0.001 mol dm–3) | M
The emf of the cell depends on the difference in concentrations of M2+ ions at the two electrodes.
The emf of the cell at 298 K is 0.059 V.
Question. To calculate the standard emf of the cell,which of the following options is correct if E° is reduction potential values?
(a) emf = E°cathode – E°anode
(b) emf = E°anode – E°cathode
(c) emf = E°anode + E°cathode
(d) None of these
Answer
A
Question. The value of DG (in kJ mol–1) for the given cell is (take 1 F = 96500 C mol–1)
(a) 3.7
(b) –3.7
(c) 10.5
(d) –11.4
Answer
D
Question. The equilibrium constant for the following reaction is
Fe2+ + Ce4+ ⇌ Ce3+ + Fe3+
(Given: E°Ce4+/Ce3+ = 1.44 V and E°Fe3+/Fe2+ = 0.68 V)
(a) 7.6 × 1012
(b) 6.5 × 1010
(c) 5.2 × 109
(d) 3.4 × 1012
Answer
A
Question. The solubility product (Ksp, mol3 dm–9) of MX2 at 298 K based on the information available for the given concentration cell is (take 2.303 × R × 298/F = 0.059)
(a) 2 × 10–15
(b) 4 × 10–15
(c) 3 × 10–12
(d) 1 × 10–12
Answer
B
Question. The solubility product of a saturated solution of Ag2CrO4 in water at 298 K if the emf of the cell Ag | Ag+ (satd. Ag2CrO4 soln) || Ag+ (0.1 M) | Ag is 0.164 V at 298 K, is
(a) 3.359 × 10–12 mol3 L–3
(b) 2.287 × 10–12 mol3 L–3
(c) 1.158 × 10–12 mol3 L–3
(d) 4.135 × 10–12 mol3 L–3
Answer
B
Case III : Read the passage given below and answer the following questions.
Nernst equation relates the reduction potential of an electrochemical reaction to the standard potential and activities of the chemical species undergoing oxidation and reduction.
Let us consider the reaction, Mn+ (aq) → nM(s)
For this reaction, the electrode potential measured with respect to standard hydrogen electrode can be given as

In the following questions , a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
Question. Assertion : Electrode potential for the electrode Mn+/Mn with concentration is given by the expression under STP conditions.
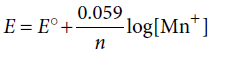
Reason : STP conditions require the temperature to be 273 K.
Answer
B
Question. Assertion : For the cell reaction,
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
voltmeter gives zero reading at equilibrium.
Reason : At the equilibrium, there is no change in concentration of Cu2+ and Zn2+ ions.
Answer
A
Question. Assertion : The Nernst equation gives the concentration dependence of emf of the cell.
Reason : In a cell, current flows from cathode to anode.
Answer
B
Question. Assertion : For concentration cell,
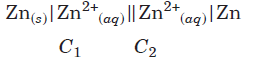
For spontaneous cell reaction, C1 < C2
Reason : For concentration cell
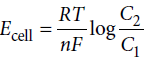
For spontaneous reaction, Ecell = +ve so, C2 > C1.
Answer
A
Question. Assertion : Increase in the concentration of copper half cell in a cell, increases the emf of the cell.
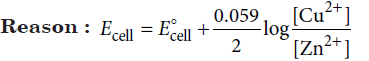
Answer
A
Case IV : Read the passage given below and answer the following questions.
The concentration of potassium ions inside a biological cell is at least twenty times higher than the outside. The resulting potential difference across the cell is important in several processes such as transmission of nerve impulses and maintaining the ion balance. A simple model for such a concentration cell involving a metal M is M(s) | M+(aq.; 0.05 molar) || M+(aq; 1 molar) | M(s)
Question. If the 0.05 molar solution of M+ is replaced by a 0.0025 molar M+ solution, then the magnitude of the cell potential would be
(a) 130 mV
(b) 185 mV
(c) 154 mV
(d) 600 mV
Answer
C
Question. The potential of an electrode change with change in
(a) concentration of ions in solution
(b) position of electrodes
(c) voltage of the cell
(d) all of these.
Answer
A
Question. The value of equilibrium constant for a feasible cell reaction is
(a) < 1
(b) = 1
(c) > 1
(d) zero
Answer
C
Question. For the above cell,
(a) Ecell = 0 ; DG > 0
(b) Ecell > 0 ; DG < 0
(c) Ecell < 0 ; DG° > 0
(d) Ecell > 0 ; DG° = 0
Answer
B
Question. What is the emf of the cell when the cell reaction attains equilibrium?
(a) 1
(b) 0
(c) > 1
(d) < 1
Answer
B
Case V : Read the passage given below and answer the following questions.
All chemical reactions involve interaction of atoms and molecules. A large number of atoms/ molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced.All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.
Question. If cathode is a Hg electrode, then the maximum weight of amalgam formed from this solution is
(Given : Atomic mass of Na = 23u and Hg = 200.59 u)
(a) 300 g
(b) 446 g
(c) 396 g
(d) 296 g
Answer
B
Question. The total number of moles of chlorine gas evolved is
(a) 0.5
(b) 1.0
(c) 1.5
(d) 1.9
Answer
B
Question. In electrolysis of aqueous NaCl solution when Pt electrode is taken, then which gas is liberated at cathode?
(a) H2 gas
(b) Cl2 gas
(c) O2 gas
(d) None of these
Answer
A