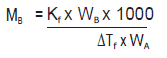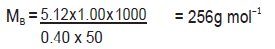VBQs Solutions Class 12 Chemistry with solutions has been provided below for standard students. We have provided chapter wise VBQ for Class 12 Chemistry with solutions. The following Solutions Class 12 Chemistry value based questions with answers will come in your exams. Students should understand the concepts and learn the solved cased based VBQs provided below. This will help you to get better marks in class 12 examinations.
Solutions VBQs Class 12 Chemistry
1 MARK QUESTIONS
Question. What do you understand by the term that Kf for water is 1.86 K kg/mol ?
Answer. It means that the freezing point of water is lowered by 1.86 K when 1 mol of non volatile solute is dissolved in 1 kg of water.
Question. Under what condition do non ideal solutions show negative deviation ?
Answer. When the new forces of interaction between the components are stronger than those in the pure components, then non ideal solutions show negative deviation.
Question. Two liquids X and Y boil at 380 K and 400K respectively, which of them is more volatile?
Answer. X is more volatile since it has low boiling point.
Question. What are minimum boiling azeotropes? Give one example.
Answer. Minimum boiling azeotropes are those which boil at lower temperature than boiling point of each component in pure state, e.g., 95.5% ethyl alcohol and 4.5% water by mass.
Question. What is the value of van’t Hoff factor for a dilute solution of
(i) K2SO4 in water
(ii) acetic acid in benzene.n
Ans .(i) 3 (ii) 1/2
Question. Why is osmotic pressure of 1 M KCl higher than 1 M urea solution ?
Answer. This is because KCl dissociates to give K+ and Cl– ions while urea being a molecular solid does not dissociate into ions in the solution.
Question. How does the molarity of a solution change with temperature?
Answer. Molarity decreases with increase in temperature as volume of solution increases with increase in temperature.
2 MARKS QUESTIONS
Question. How is that measurement of osmotic pressure is more widely used for determining molar masses of macromolecules than the elevation in boiling point or depression in freezing point of their solutions?
Answer. The osmotic pressure method has the advantage over elevation in boiling point or depression in freezing point for determining molar masses of macromolecules because
1. Osmotic pressure is measured at the room temperature and the molarity of solution is used instead of molality.
2. Compared to other colligative properties, its magnitude is large even for very dilute solutions.
Question. Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile (CH3CN) when 6.5 g of C9H8O4 is dissolved in 450 g of CH3CN .
Answer. Mass of solution = 6.5g + 450g = 456.5g
Mass % of aspirin = Mass of aspirin
=6.5/456.5 X 100 = 1.424% Mass of solution x 100
Question. Suggest the most important type of intermolecular interaction in the following pairs :
i) N-hexane and n-octane
ii) methanol and acetone
Answer. i) Dispersion or London forces as both are non-polar.
ii) Dipole-dipole interactions as both are polar molecules.
Question. State Henry’s l aw. What is the significance of KH ?
Answer. Henry’s Law: It states that “the partial pressure of the gas in vapour phase (p) is directly proportional to the mole fraction of the gas (x) in the solution” , and is expressed as : p=KH x where,KH is the Henry’s Law constant Significance of KH : Higher the value of Henry’s law constant KH ,the lower is the solubility of the gas in the liquid .
3 MARK QUESTIONS
Question. a) Why is an increase in temperature observed on mixing chloroform and acetone?
b) Why does sodium chloride solution freeze at a lower temperature than water?
Answer. a) The bonds between chloroform molecules and molecules of acetone are dipole-dipole interactions but on mixing, the chloroform and acetone molecules, they start forming hydrogen bonds which are stronger bonds resulting in the release of energy. This gives rise to an increase in temperature.
b) When a non- volatile solute is dissolved in a solvent, the vapour pressure decreases. As a result, the solvent freezes at a lower temperature.
Question. Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litre of water such that its osmotic pressure is 0.75 atm at 27⁰C.
Answer.
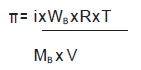
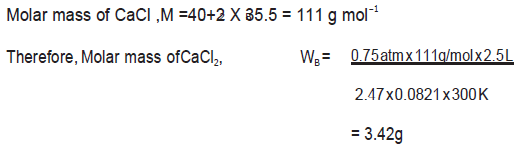
Question. Non-ideal solution exhibit either positive or negative deviations from Raoult’s law. What are these deviation and why are they caused? Explain with one example for each type.
Answer. When the vapour pressure of a solution is either higher or lower than that predicted by Raoult’s law, then the solution exhibits deviation from Raoult’s law. These deviation are caused when solute – solvent molecular interactions A – B are either weak or stronger than solvent – solvent A – B or solute – solute B – B molecular interactions. Positive deviations : When A – B molecular interactions are weaker than A – A and B – B molecular interaction . For example, a mixture of ethanol and acetone.
Negative deviations: When A – B molecular interaction are stronger than A – A and B – B molecular interaction.
For example, a mixture of chloroform and acetone.
Question. The molar freezing point depression constant for benzene is 4.90K kgmol–1. Selenium exists as polymer SeX. When 3.26 gm of Se is dissolved in 226gm of benzene, the observed freezing point is 0.1120C
lower than for pure benzene. Decide the molecular formula of Selenium.(At.wt. of selenium is 78.8 g mol‐1)
Answer.

Question. A solution of glycerol (C3H8O3) in water was prepared by dissolving some glycerol in 500g of water. This solution has a boiling point of 100.42C while pure water boils at 100-C. What mass of glycerol was dissolved to make the solution ? (Kb of water = 0.512 K kg/mol)
Answer. ΔTb = 100.42°C‐ 100°C = 0.42°C or 0.42K; WA = 500g ; Kb = 0.512 K kg / mol ;MB = 92 g /mol Substituting these values in the expressions,
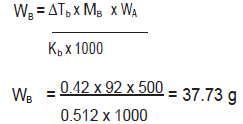
5 MARKS QUESTION
Question. a) Calculate the molarity of a sulphuric acid solution in which the mole fraction of water is 0.85.
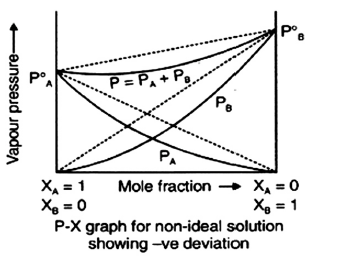
b) The graphical representation of vapour pressure of two component system as a function of composition is given alongside.
i) Are the A – B interactions weaker, stronger or of the same magnitude as A – A and B – B
ii) Name the type of deviation shown by this system from Raoult’s law.
iii) Predict the sign of ΔmixH for this system.
iv) Predict the sign of ΔmixV for this system.
v) Give an example of such a system.
vi) What type of a zeotrope will this system form, if possible ?
Answer.
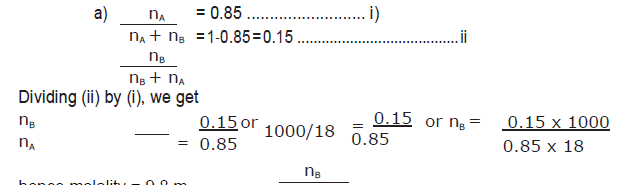
hence molality = 9.8 m
b) i) Stronger
ii) Negative deviation
iii) Negative
iv) Negative
v) 20% acetone and 80% chloroform by mass
vi) maximum boiling azeotrope
Question. a) State Raoult’s Law for a solution containing volatile components.
How does Raoult’s law become a special case of Henry’s Law?
b) 1.00 g of a non‐electrolyte solute dissolved in 50 g of benzene lowered the freezing point of a benzene by 0.40 K. Find the molar mass of the solute. (Kf for benzene = 5.12 K kg mol–1)
Answer. a) For a solution of volatile liquids , Raoult’s law states that the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution, i.e., pA∝ xA
OR
pA = poA xA
According to Henry’s Law , the partial pressure of a gas in vapour phase (p) is Directly proportional to mole fraction (x) of the gas in the solution. i.e., p = KHx on comparing it with Raoult’s Law it can be seen that partial pressure of the volatile component or gas is directly proportional to its mole fraction in solution i.e; p ∝x
only the proportionality constant K differs from p0 . Thus, it becomes a special case of Henry’s law in which K = po .
b) Substituting the values of various terms involved in equation