Students should refer to Worksheets Class 10 Science Carbon and Its Compound Chapter 4 provided below with important questions and answers. These important questions with solutions for Chapter 4 Carbon and Its Compound have been prepared by expert teachers for Class 10 Science based on the expected pattern of questions in the class 10 exams. We have provided Worksheets for Class 10 Science for all chapters on our website. You should carefully learn all the important examinations questions provided below as they will help you to get better marks in your class tests and exams.
Carbon and Its Compound Worksheets Class 10 Science
Question. The structural formula of ethyl ethanoate
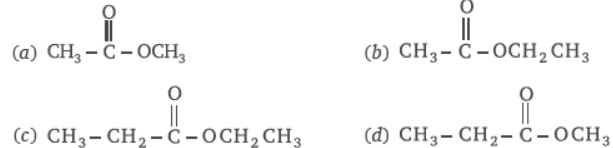
Answer
B
Question. Which of the following is used to oxidise ethanol to ethanoic acid?
(a) Alkaline KMnO4
(b) Conc. H2SO4
(c) Acidified K2Cr2O7
(d) All of above
Answer
D
Question. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Answer
C
Question. Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Answer
B
Question. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer
B
Question. The compound which gives a brisk effervescence with sodium metal and not with sodium hydrogen carbonate is
(a) ethanol
(b) ethanoic acid
(c) both ethanoic acid and ethanol
(d) none of these
Answer
A
Question. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Answer
D
Question. Vinegar is a solution of
(a) 30% – 40% acetic acid in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 15% – 20% acetic acid in water
Answer
C
Question. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer
B
Question. Identify the product formed when methane reacts with chlorine in the presence of sunlight is
(a) C2Cl6
(b) CH3Cl
(c) CHCl4
(d) None of these
Answer
B
Question. The heteroatoms present is
CH3 – O – CH2 – CH2 (Br)
(a) oxygen
(b) carbon
(c) hydrogen
(d) bromine
Answer
D
Question. Drinking alcohol and driving may cause serious accidents. To discourage this, police randomly test drivers for alcohol using a breath analyser. The breath analyser works because
(a) Alcohol makes the breath dry and the machine registers moisture
(b) Alcohol makes the breath hotter which changes the machine reading
(c) Alcohol causes more saliva which the machine checks.
(d) Alcohol in the breath cause a chemical change registered by the machine.
Answer
B
Question. Acetic acid was added to a liquid X kept in a test tube. A colourless and odourless gas Y was evolved. The gas was passed through lime water which turned milky. It was concluded that:
(a) Liquid X is sodium hydroxide and the gas Y is CO2
(b) Liquid X is sodium carbonate and the gas Y is CO2
(c) Liquid X is sodium acetates and the gas Y is CO2
(d) Liquid X is sodium chloride and the gas Y is SO2.
Answer
B
Question. Tertiary butane gets oxidised with oxidising agents like alkaline KMNO4 to
(a) Isobutane
(b) Ter-butyl alcohol
(c) Secondary-propyl alcohol
(d) All of above
Answer
B
Question. The number of single and double bonds present in benzenes are
(a) 9 and 6
(b) 9 and 3
(c) 12 and 3
(d) 12 and 6
Answer
B
Question. The isomeric pair is
(a) ethane and propane
(b) propane and butane
(c) ethane and ethane
(d) butane and 2-methyl propane
Answer
D
Question. The substance not responsible for the hardness of water is
(a) sodium nitrate
(b) calcium hydrogen carbonate
(c) calcium carbonate
(d) magnesium carbonate
Answer
A
Question. The by product of soap is
(a) isoprene
(b) glycerol
(c) butene
(d) ethylene glycol
Answer
B
Question. Covalent compounds
(a) have high melting and boiling points
(b) are mostly soluble in water
(c) are formed between atoms of metals and non-metals
(d) are formed by the sharing of electrons in the bonding atoms.
Answer
D
Question. Which of the following can be used for the denaturation of ethyl alcohol?
(a) Methyl alcohol
(b) Pyridines
(c) Copper sulphate
(d) All of above
Answer
D
Question. According to IUPAC system, the correct name of the organic compound is
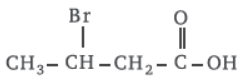
(a) 2-bromobutanoic acid
(b) 2-bromobutysis acid
(c) 3-bromobutanoic acid
(d) 3-bromo-2-hydroxybutan-2-one
Answer
C
Question. Soaps are formed by saponification of
(a) alcohols
(b) glycosides
(c) simple esters
(d) carboxylic acids
Answer
C
Question. The correct electron dot structure of a water molecule is

Answer
C
Question. Which is denatured spirit?
(a) ethanol only
(b) ethanol and methanol (50%)
(c) ethanol and methanol (5%)
(d) methanol only
Answer
C
Question. For gas welding used for welding broken pieces of iron, we normally use a mixture of
(a) Ethane and oxygen
(b) Ethene and oxygen
(c) Ethyne and oxygen
(d) Ethene and air
Answer
A
Question. Bromine reacts with saturated hydrocarbon at room temperature in the
(a) absence of sunlight
(b) presence of water
(c) presence of sunlight
(d) presence of hydrochloric acid
Answer
C
Question. Identify the correct way of numbering an organic compound (according to IUPAC)
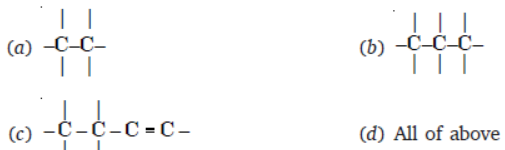
Answer
A
Question. Identify the functional group present in the following compound

(a) aldehyde
(b) bromine
(c) carboxylic
(d) both bromine and carboxylic group
Answer
D
Question. The upper and lower homologue of C2H5OH are respectively
(a) methyl alcohol and butyl alcohol
(b) ethyl alcohol and propyl alcohol
(c) butyl alcohol and propyl alcohol
(d) propyl alcohol and methyl alcohol
Answer
D
Question. Which is not true about homologous series?
(a) They have same general formula.
(b) They differ from other by CH3 group.
(c) They have same functional group.
(d) They have same chemical properties.
Answer
B
Question. Name the following aromatic compound

(a) toluene
(b) aniline
(c) phenol
(d) furan
Answer
A
Question. Ethanoic acid was added to sodium carbonate solution and the gas evolved was tested with a burning splinter. The following four observations were reported. Identify the correct observation.
(a) The gas burns with pop sound and the flame gets extinguished
(b) The gas does not burn but the splinter burns with pop sound
(c) The flame extinguishes and the gas does not burn
(d) The gas burns with a blue flame and the splinter burns brightly
Answer
C
Question. Which of the following is not a straight chain?
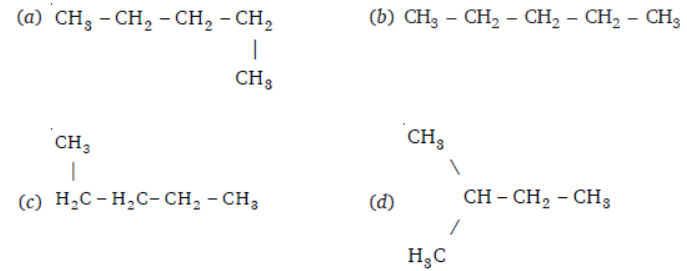
Answer
D
Question. The general formula for alkanes is CnH2n+1–CHO. The value of ‘n’ for the first member.
(a) 1
(b) 0
(c) 0.5
(d) 1.1
Answer
B
Question. An organic compound ‘X’ has the molecular formula C3H6O2. It has a pleasant smell but does not turn blue limus red. It has structural formula
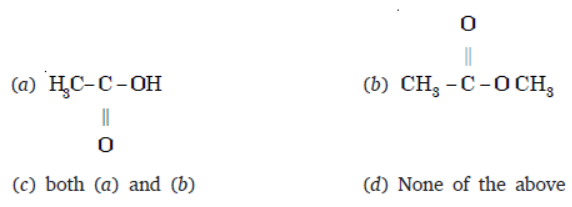
Answer
B
Question. Identify the compound that undergoes bromination reaction:
Answer
D
Question. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer : Carbon form large number of compounds due to the following properties:
(a) Catenation → Carbon shows the property of catenation that is the ability to form bonds with other carbon atoms forming long chains both branched and unbranched chains, and even rings.
(b) Tetravalency → Carbon has valency 4, it is capable of bonding with 4 other carbon atoms or atoms of other non-covalent elements, giving rise to compounds with specific properties depending on the elements present in the compound.
(c) Isomerism → Carbon compounds show the property of isomerism that is compounds having same molecular formula but different structural formula.
Question. What would be the formula and electron dot structure of cyclopentane?
Answer : The formula of cyclopentane is C5H10.
The electron dot structure is

Question. What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer : The electron dot structure of CO2 is

Question. People use variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Answer : Soap lowers the surface tension of water. The long chain non-ionic hydrocarbon group in soap gets attached to the oil or grease droplets and loosens them from the fibres of cloth along with the dirt. However this loosening is insufficient to remove the grease with dirt completely. Hence the clothes are agitated to remove the grease droplets completely.
Question. How would you name the following compounds:

Answer : (i) Bromo ethane (ii) Methanal
(iii) Hex-1-yne
Question. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer : Conversion of ethanol to ethanoic acid is an oxidation reaction because oxygen is added to ethanol to convert it to ethanoic acid.
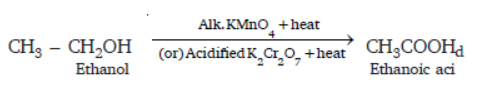
In the above reaction alk. KMnO4/acidified K2Cr2O7 add oxygen to ethanol hence they are called oxidising agent.
Question. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer : If air is used, incomplete combustion will take place giving a sooty flame and less heat is produced. When pure oxygen is used ethyne burns completely producing large amount of heat and blue flame. This heat is sufficient for a metal to melt and welding is done.
Question. How many structural isomers can you draw for pentane?
Answer : Three structural isomers can be drawn from pentane.
Pentane : C5H12
Question. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer : (a) Acid test: Reaction with carbonates/hydrogen carbonates.
Take samples of alcohol and carboxylic acid in 2 test tubes, and add sodium carbonate or sodium bicarbonate solution to each. The compound which will produce brisk effervescence of CO2 gas will be acid.
(b) Alcohol test: Take small amount of ethanol and ethanoic acid in test tube A and B. Add 5% solution of alkaline potassium permanganate drop by drop to this solution and warm the test tube.
The colour of potassium permanganate will disappear in test tube containing alcohol.
Question. What are oxidising agents?
Answer : The compounds which add oxygen to other substance are known as oxidising agent.
For example, alkaline potassium permanganate solution and acidified potassium dichromate, both can convert alcohol into carboxylic acid, i.e., ethanoic acid.
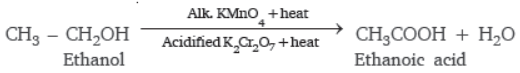
Question. Would you be able to check if water is hard by using detergent?
Answer : No, because detergent forms lather in both, hard and soft water.
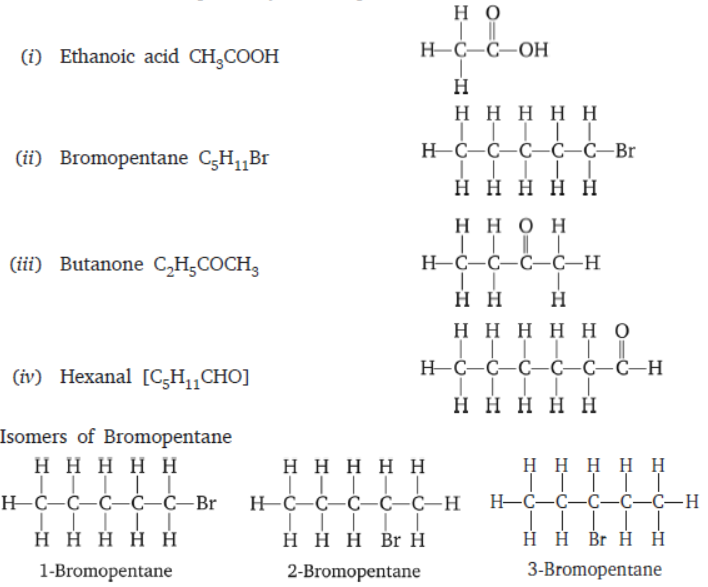
Question. Draw the structures for the following compounds.
(i) Ethanoic acid (ii) Bromopentane
(iii) Butanone (iv) Hexanal
Are structural isomers possible for bromopentane?
Answer :
Question. Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Answer : (b) 7 covalent bonds.
Question. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Answer : (d) ketone.
VERY SHORT ANSWER TYPE QUESTIONS
Question. What is a hydrocarbon?
Answer : It is a compound of hydrogen and carbon.
Question. Give different forms in which carbon occurs in nature.
Answer : Carbon occurs in free form e.g., graphite and diamond in combined form like carbon dioxide, carbonates, etc.
In earth’s crust–0.02% and in atmosphere–0.03%.
Question. Name two types of hydrocarbon.
Answer : Hydrocarbon – Saturated and unsaturated.
Question. What is an homologous series? Explain with an example.
Answer : It is a group of members of same class of organic compound having similar chemical properties, they have same general formula.
They have same functional group, when arranged in the ascending order of molecular mass they differ by 14 a.m.u. or —CH2 group.
Question. What is addition reaction? Give one example.
Answer : The process of adding hydrogen across the double bonds of unsaturated hydrocarbons is called addition reaction.
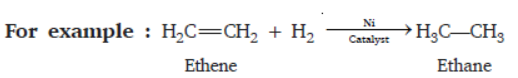
Question. What are covalent bonds?
Answer : Bond which are formed by sharing of a pair of electrons between two atoms is called covalent bonds.
Question. Name two allotropes of carbon.
Answer : Two allotropes are – Crystalline and amorphous
Crystalline form – Diamond and graphite.
Amorphous form – Charcoal, coal, coke.
Question. Why covalent compounds have low melting and boiling points?
Answer : As the bond is formed by sharing of electrons between two atoms. Intermolecular forces are small between the covalent compounds. These bonds break easily.
Question. Define oxidising agents.
Answer : Some substances are capable of adding oxygen to others. These substances are known as oxidising agents.
Example: alkaline KMnO4 and acidified K2Cr2O7.
Question. Give the reaction to show how alcohol is converted into carboxylic acid.
Answer :
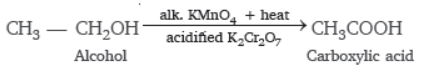
Question. Identify the compound
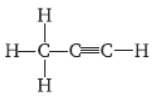
Answer : Propyne.
Question. Give two properties of ethanol.
Answer : (a) Liquid at room temperature
(b) Soluble in H2O in all proportions
Question. Give the formula for the functional group of aldehyde.
Answer :
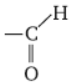
Question. What are heteroatoms?
Answer : An element or group of elements which replaces one or more hydrogen (H) atoms from hydrocarbon, such that valency of carbon remains satisfied.
Example: CH4 ⎯→ CH3 – OH
Hence, —OH is a heteroatom.
Question. Define catalyst.
Answer : Catalyst are substances that cause a reaction with a change in rate of reaction, without itself undergoing any change.
Example: Micelle acts as a catalyst to convert unsaturated hydrocarbon into saturated hydrocarbon.
Question. Complete the following reaction:
Answer :

Question. Give the full form of IUPAC.
Answer : IUPAC → International Union of Pure and Applied Chemistry.
Question. Give the IUPAC name and write the functional group present in vinegar.
Answer : Vinegar IUPAC name is acetic acid CH3COOH
Functional group –COOH
Question. How can esters be converted into soap?
Answer : By saponification reaction, by adding/reacting ester with NaOH.
Question. How can we convert CH3CH2OH into C2H4 ?
Answer : By adding conc. sulphuric acid into it which acts as dehydrating agent and removes water from it.
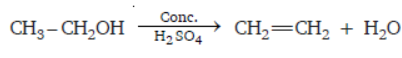
SHORT ANSWER TYPE QUESTIONS
Question. What is the reactive site in the given hydrocarbon? Write its name.
H3C—CH2—CH==CH—CH3
Answer : The reactive site is at a place where double bond is present.
Name of the compound is 2-pentene.
Question. What is the difference in the number of carbon and hydrogen atoms between two successive members of a homologous series? Also give the difference in their atomic masses.
Answer : The difference is of 1 carbon and two hydrogen atoms i.e., —CH2 and mass difference is 14 a.m.u.
Question. Why acetic acid is called glacial acetic acid?
Answer : Acetic acid has very low melting point i.e. 290 K, hence it freezes during winters in cold countries. So it is called glacial acetic acid.
Question. Write the structural formula for bromopentane and ethanoic acid.
Answer :

Question. How does ethanoic acid react with carbonates and hydrogen carbonates? Show it with the equation.
Answer : Ethanoic acid reacts with carbonates and hydrocarbonates to form salt, CO2 and H2O.
The salt formed is sodium acetate.
2CH3COOH + Na2CO3 ⎯→ 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 ⎯→ CH3COONa + H2O + CO2
Question. Draw the structures of two isomers of butane.
Answer : Butane C4H10
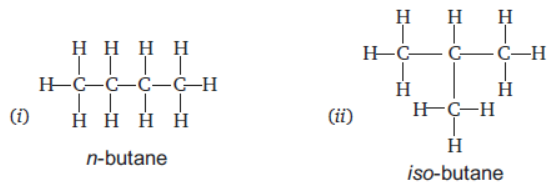
Question. A student burns a hydrocarbon in air and obtains sooty flame. Give two reasons for this observation.
Answer : Sooty flame could be obtained due to
(i) Incomplete combustion of saturated hydrocarbons.
(ii) Combustion of unsaturated hydrocarbon.
Question. Give difference between soap and detergent.
Answer :
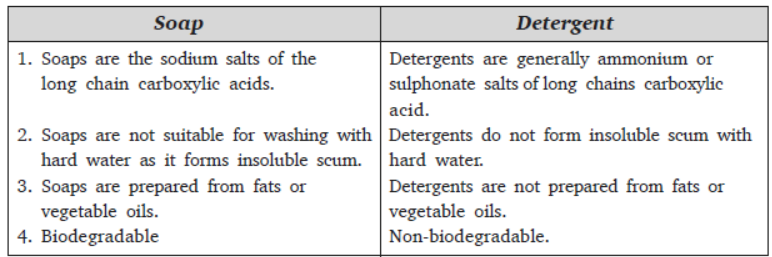
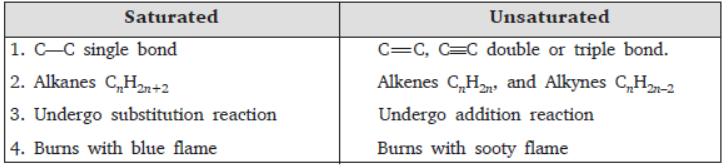
Question. Differentiate between saturated and unsaturated hydrocarbons. Give one example for each.
Answer :

Question. Write the general formula for each of the following hydrocarbons and give one example for each.
(i) Alkene (ii) Alkyne
Answer : (i) Alkene CnH2n e.g., C2H4 ethene
(ii) Alkyne CnH2n–2 e.g., C2H2 ethyne
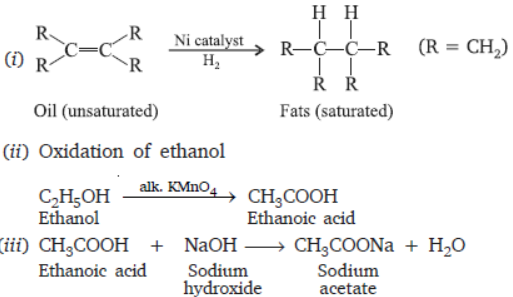
Question. Write the chemical equations for the following reactions:
(i) Conversion of oils into fats (ii) Oxidation of ethanol
(iii) Ethanoic acid with sodium hydroxide.
Answer :
Question. Name the functional groups of the following:

Answer : (a) Chloro (Halogen)
(b) Carboxylic acid
(c) Ketone
(d) Alcohol
Question. Explain substitution reaction with example.
Answer : The reaction of saturated hydrocarbon with chlorine in which each hydrogen atom slowly gets substituted with chlorine atom is called substitution reaction.
CH4 + Cl2 ⎯⎯→ CH3Cl + HCl
Methane Chlorine Methyl Hydrochloric
Question. Diamond and graphite show different physical properties although they are made up of carbon and shows same chemical properties. What is this property called?
Answer : This property is allotropy.
The physical properties are different because the carbon-carbon bonding in both the cases varies. In diamond one carbon atom is bonded with four other carbon atoms with strong covalent bond so it is hard, while in case of graphite each carbon forms two strong bonds with other two carbon atoms and one weak bond is formed with third carbon atom and forms hexagonal rings which slide over each other, so it is soft.
Question. What is denatured alcohol?
Answer : When ethanol is mixed with methanol or some poisonous substances such as copper sulphate, pyridine which makes it unfit for drinking such alcohol is called denatured alcohol.
Question. What is esterification and give its uses?
Answer : It is the reaction in which esters are formed by reacting carboxylic acid with alcohol in the presence of concentrated sulphuric acid.
Carboxylic acid + alcohol ⎯⎯→ Ester + water
C2H5OH + CH3COOH ⎯⎯→ CH3COOC2H5 + H2O
Ethanol Ethanoic acid Ester
Uses:(i) It is used as flavour in ice-cream and sweets.
(ii) It is a sweet smelling substance.\
LONG ANSWER TYPE QUESTIONS
Question. An organic compound ‘A’ is widely used as a preservative in pickles and has a molecular formula C2H4O2. This compound reacts with ethanol to form a sweet smelling compound ‘B’.
(a) Identify the compound ‘A’.
(b) Write the chemical equation for its reaction with ethanol to form compound ‘B’.
(c) How can we get compound ‘A’ and ‘B’?
(d) Which gas is obtained when compound ‘A’ reacts with washing soda? Give the equation.
(e) Write an equation to obtain ‘A’ back from ‘B’.
Answer 🙁a) ‘A’ is CH3COOH acetic acid.
(b) CH3COOH + C2H5OH ⎯⎯→ CH3COOC2H5 + H2O
(c) We can get compound A back by the process of saponification.
(d) A + washing soda ⎯⎯→ CO2 gas is produced
2CH3COOH + Na2CO3 ⎯⎯→ 2CH3COONa + H2O + CO2
(e) Saponification
CH3COOC2H5 ⎯N⎯aO⎯H→ C2H5OH + CH3COONa
Question. Identify the compound A, B, C, D, and E in the following reaction:
(a) CH3CH2OH → Ⓐ CH3COOH
(b) CH3CH2OH + CH3COOH → conc. Ⓑ + H2O H2SO4
(c) B + NaOH → C2H5OH + Ⓒ
(d) D + Na2CO3 → CH3COONa + E + H2O
(e) E + Ca(OH)2 → Ⓕ + H2O
white ppt.
Answer : (a) A = Alkaline KMnO4 or acidified K2Cr2O7
(b) B = CH3COOC2H5
(c) C = CH3COONa
(d) D = CH3COOH; E = CO2
(e) E = CO2; F = CaCO3
Question. What are soaps? Explain the mechanism of the cleansing action of soaps? Soaps form scum with hard water. Explain why? How this problem is overcome by use of detergents?
Answer : Soaps are sodium salts of fatty acids. It is biodegradable and shows cleansing action by removing dirt.
Mechanism of cleansing action: Soap has molecules with tadpole like structure.

Its head is made of Na+ ion which is hydrophilic and long tail is made up of hydrocarbon chain which is hydrophobic, it attracts the dirt and removes it. When soap is added to water it forms micelles and helps in removing the dirt which sticks to the carbon chain. On rinsing the cloth with water it helps in removing the dirt, as Na+ is hydrophilic. It attracts water and carries its tail entangled with dirt and flows away with water.
Scum formation: Soaps form scum with hard water because hard water has salts of calcium and magnesium which react with soap to form insoluble compound called scum.
In case of detergents, the salts present in hard water does not react with the molecules of detergent to form insoluble compound called scum, but the molecules of detergent remain as it is and helps in the cleansing action.
Question. (a) What do you mean by allotropy?
(b) What is isomerism?
(c) Give one example of homologus series, give two properties of it.
(d) What is the full form of IUPAC?
Answer : (a) Allotropy: It is the property of an element in which element show same chemical properties but different physical properties, due to difference in the bonding of atoms.
Example: Diamond and graphite are having same chemical properties but they look/appear to be physically different as the bonding in both differs.
(b) Isomerism: It is the property of hydrocarbons which show same molecular formula but exhibits different structural formulae.
Example: Butane
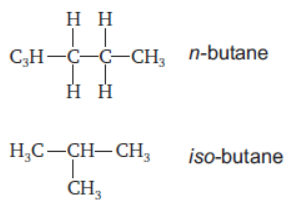
Both of them show different properties.
(c) Homologous series: When the members of a hydrocarbon family obey same general formula they are said to be in homologous series. When the members are arranged in increasing order of their molecular masses:
Example: Alkane – CnH2n+2
CH4 — Methane
C2H6 — Ethane
C3H8 — Propane
C4H10 — Butane
Properties:
(i) The difference between two consecutive members of homologous series is of —CH2 and mass 14 a.m.u.
(ii) They all show same chemical properties and slight gradation in their physical properties.
(d) IUPAC: International Union of Pure and Applied Chemistry.
Question. (a) What are hydrocarbons?
(b) Give difference between saturated and unsaturated hydrocarbons.
(c) Why does carbon form large number of compounds?
Answer : (a) Hydrocarbons – A compound of carbon and hydrogen.
(b)
(c) Carbon forms large number of compounds due to
(i) Catenation – Self linking property which leads to long straight chains, branched chains and cyclic chains.
(ii) Isomerism – Compound of carbon can exist in more than one structural formula but has same molecular formula.
(iii) Tetravalency – To acquire noble gas configuration, carbon shares its outer electrons with other elements, thus form covalent bond with other elements.
Question. A, B, C are members of homologous series their melting points are –183°C, –138°C, 130°C respectively. Among these
(i) Which member will have least number of carbon atoms?
(ii) Which member will have maximum number of carbon atoms?
Answer : (i) A will have least number of carbon atoms.
(ii) C will have maximum number of carbon atoms.
Question. A hydrocarbon compound A is active ingredient of wine and cough syrup. A on oxidation with acidified K2Cr2O7 forms compound B. Identify the compound A and B and write the chemical equations involved.
Answer : A is ethanol, C2H5OH
B is ethanoic acid, CH3COOH
Equation : C2H5OH acid K2Cr2O7 ⎯⎯⎯⎯⎯⎯→ CH3COOH
Question. Write an activity to show the acidic nature of ethanol. Give the chemical equation of the reaction taking place.
Answer : Take ethanol in a test tube and drop a small piece of sodium about the size of a grain of rice into it. The reaction evolves a colourless gas which is hydrogen.
Hydrogen gas can be tested by bringing a burning splinter/match stick near the mouth of the test tube, it burns with the popping sound.
This activity proves that ethanol like other acids release H2 gas
2Na + 2CH3CH2OH ⎯⎯→ 2CH3CH2ONa + H2
Question. A compound ‘X’ has molecular formula C2H6O is saturated hydrocarbons and is a very good solvent. How can you convert it into unsaturated hydrocarbon? Identify X and show its conversion with the help of equation.
Answer : ‘X’ is CH3—CH2OH ethanol. It can be made unsaturated by heating it with conc.
H2SO4 which is a dehydrating agent removes water from it, thereby forming ethene.
Question. Take about 20 ml of castor oil in a beaker. Add 30 ml of 20% sodium hydroxide solution. Heat the mixture with continuous stirring for a few minutes till the mixture thickens. Add 5–10 g of common salt to this. Stir the mixture well, allow it to cool, soaps is obtained.

(a) Why do we use common salt to make soap?
(b) What will happen if you will add the above made soap solutions to the following test tubes A, B, and C.
(c) Can we use potassium hydroxide instead of sodium hydroxide.
Answer : (a) Salt: NaCl is added while making soap, because it will help the reaction to occur faster and adds sodium ion to increase the reaction rate.
(b) In test tube A: Soap + Oil ⎯→ Lather/foam is formed. Carboxylic chain dissolves in oil.
In test tube B: Soap + Hard water ⎯→ Insoluble compound called scum is formed.
In test tube C: Soap + soft water ⎯→ Froth is formed.
(c) Yes, we can use potassium hydroxide to prepare soap.



