Students should refer to Worksheets Class 12 Chemistry Aldehydes Ketones and Carboxylic Acids Chapter 12 provided below with important questions and answers. These important questions with solutions for Chapter 12 Aldehydes Ketones and Carboxylic Acids have been prepared by expert teachers for Class 12 Chemistry based on the expected pattern of questions in the Class 12 exams. We have provided Worksheets for Class 12 Chemistry for all chapters on our website. You should carefully learn all the important examinations questions provided below as they will help you to get better marks in your class tests and exams.
Aldehydes Ketones and Carboxylic Acids Worksheets Class 12 Chemistry
Question. Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
Ethanal, Propanal, Propanone, Butanone
(a) Butanone < Propanone < Propanal < Ethanal
(b) Propanone < Butanone < Ethanal > Propanal
(c) Propanal < Ethanal < Propanone < Butanone
(d) Ethanal < Propanal < Propanone < Butanone
Answer
A
Question. Propanal on treatment with dilute sodium hydroxide gives
(a) CH3CH2CH2CH2CH2CHO
(b) CH3CH2CH(OH)CH2CH2CHO
(c) CH3CH2CH(OH)CH(CH3)CHO
(d) CH3CH2COOH
Answer
C
Question. Which of the following names of the organic compounds is not correctly written?
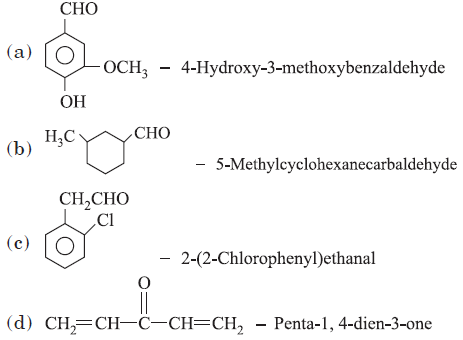
Answer
B
Question. Various products formed on oxidation of 2,5-dimethylhexan-3-one are
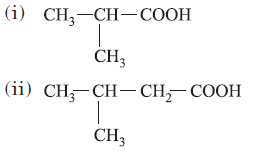
(iii) CH3COOH
(iv) HCOOH
(a) (i) and (iii)
(b) (i), (ii) and (iii)
(c) (i), (ii), (iii) and (iv)
(d) (iii) and (iv)
Answer
C
Question. Which of the following IUPAC names is not correctly matched?


Answer
A
Question. a-Hydroxypropanoic acid can be prepared from ethanal by following the steps given in the sequence.
(a) Treat with HCN followed by acidic hydrolysis.
(b) Treat with NaHSO3 followed by reaction with Na2CO3.
(c) Treat with H2SO4 followed by hydrolysis.
(d) Treat with K2Cr2O7 in presence of sulphuric acid.
Answer
A
Question. Which of the following statements is incorrect?
(a) FeCl3 is used in the detection of phenols.
(b) Fehling solution is used in the detection of glucose.
(c) Tollens’ reagent is used in the detection of unsaturation.
(d) NaHSO3 is used in the detection of carbonyl compounds.
Answer
C
Question. Which of the following reagents are not correctly matched with the reaction?

Answer
B
Question. Identify the products (X) and (Y) in the given reaction :

(a) X = Acetophenone, Y = m-Nitroacetophenone
(b) X = Toluene, Y = m-Nitroacetotoluene
(c) X = Acetophenone, Y = o and p-Dinitroacetophenone
(d) X = Benzaldehyde, Y = m-Nitrobenzaldehyde
Answer
A
Question. The condensation product of benzaldehyde and acetone is
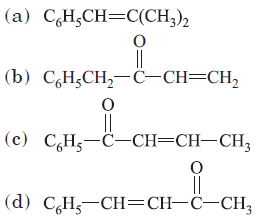
Answer
D
Question.

Structure of A and type of isomerism in the above reaction are
(a) Prop-1-en-2-ol, metamerism
(b) Prop-1-en-1-ol, tautomerism
(c) Prop-2-en-2-ol, geometrical isomerism
(d) Prop-1-en-2-ol, tautomerism.
Answer
D
Question. Which among the following is most reactive to give nucleophilic addition?
(a) FCH2CHO
(b) ClCH2CHO
(c) BrCH2CHO
(d) ICH2CHO
Answer
A
Question. Which of the following reactions does not occur?
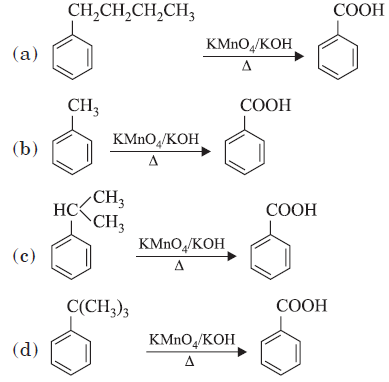
Answer
D
Question. Which of the following is the most reactive isomer?
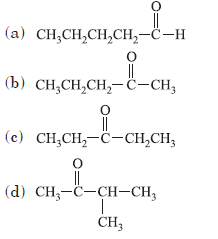
Answer
A
Question.
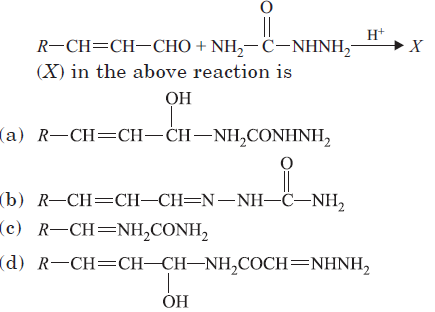
Answer
B
Question. To differentiate between pentan-2-one and pentan-3-one a test is carried out. Which of the following is the correct answer?
(a) Pentan-2-one will give silver mirror test
(b) Pentan-2-one will give iodoform test.
(c) Pentan-3-one will give iodoform test
(d) None of these.
Answer
B
Question. A ketone ‘A’ (C4H8O), which undergoes a haloform reaction, gives a compound ‘B’ on reduction. ‘B’ on heating with sulphuric acid gives a compound ‘C’ which forms mono-ozonide
‘D’. ‘D’ on hydrolysis with zinc dust gives only, ‘E’.
Identify the correct statement.
(a) A is butan-2-one; B is butan-2-ol.
(b) B is but-2-ene; C is acetaldehyde.
(c) D is acetaldehyde; E is butan-2-ol.
(d) B is butan-1-one; D is but-2-ene.
Answer
A
Question. In the following sequence of reaction, the final product (Z) is
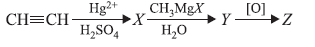
(a) ethanal
(b) propan-2-ol
(c) propanone
(d) propan-1-ol
Answer
C
Question. What happens when a carboxylic acid is treated with lithium aluminium hydride?
(a) Aldehyde is formed.
(b) Primary alcohol is formed.
(c) Ketone is formed.
(d) Grignard reagent is formed.
Answer
B
Question. Addition of water to alkynes occurs in acidic medium and in the presence of Hg2+ ions as a catalyst. Which of the following products will be formed on addition of water to but-1-yne under these conditions?
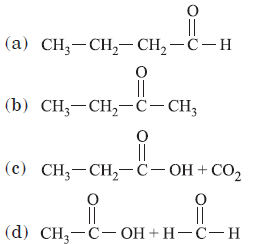
Answer
B
Question. Propanone can be prepared from ethyne by
(a) passing a mixture of ethyne and steam over a catalyst, magnesium at 420°C
(b) passing a mixture of ethyne and ethanol over a catalyst zinc chromite
(c) boiling ethyne with water and H2SO4
(d) treating ethyne with iodine and NaOH.
Answer
A
Question. Compound (X) with molecular formula C3H8O is treated with acidified potassium dichromate to form a product (Y) with molecular formula C3H6O. (Y) does not form a shining silver mirror on warming with ammoniacal AgNO3.(Y) when treated with an aqueous solution of NH2CONHNH2. HCl and sodium acetate to give a product (Z). The structure of (Z) is

Answer
B
Question. A compound (X) having molecular formula C4H8O2 is hydrolysed by water in presence of an acid to give a carboxylic acid (Y) and an alcohol (Z). (Z) on oxidation with chromic acid gives (Y).(X), (Y) and (Z) are
X Y Z
(a) CH3COOCH3 CH3COOH CH3OH
(b) CH3COOC2H5 CH3COOH C2H5OH
(c) C2H5COOCH3 C2H5COOH C2H5OH
(d) CH3COOC2H5 C2H5COOH CH3OH
Answer
B
Question. Match the column I with column II and mark the appropriate choice.
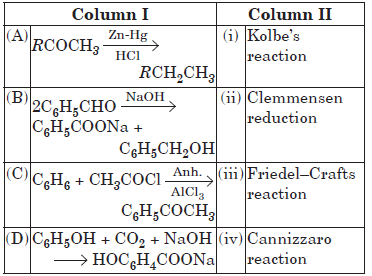
(a) (A) → (ii), (B) → (iv), (C) → (iii), (D) → (i)
(b) (A) → (i), (B) → (iii), (C) → (ii), (D) → (iv)
(c) (A) → (iii), (B) → (ii), (C) → (i), (D) → (iv)
(d) (A) → (iv), (B) → (i), (C) → (ii), (D) → (iii)
Answer
A
Question. What are the correct steps to convert acetaldehyde to acetone?
(a) CH3MgBr, H2O, Oxidation
(b) Oxidation, Ca(OH)2, Heat
(c) Reduction, KCN, Hydrolysis
(d) Oxidation, C2H5ONa, Heat
Answer
B
Question. Ketones
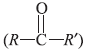
can be obtained in one step by (where R and R′ are alkyl groups)
(a) hydrolysis of esters
(b) oxidation of primary alcohols
(c) oxidation of secondary alcohols
(d) reaction of alkyl halides with alcohols.
Answer
C
Question. Hydrocarbons are formed when aldehydes and ketones are reacted with amalgamated zinc and conc. HCl. The reaction is called
(a) Cannizzaro reaction
(b) Clemmensen reduction
(c) Rosenmund reduction
(d) Wolff-Kishner reduction.
Answer
B
Question. The best oxidising agent for oxidation of

(a) Baeyer’s reagent
(b) Tollen’s reagent
(c) Schiff’s reagent
(d) Acidified dichromate.
Answer
B
Question. Carboxylic acids do not undergo Friedel Craft’s reaction because
(a) —COOH group is meta directing
(b) —COOH group is resonance stabilised
(c) carboxyl group is deactivating and gets bonded to Friedel Craft’s catalyst
(d) all of above.
Answer
C
Question. Identify reactant (X) in the given reaction sequence.

(a) CH3MgCl
(b) CH3COCl + Mg
(c) MgCl2
(d) CH3CH2MgCl
Answer
A
Question. In the following reaction, product (P) is
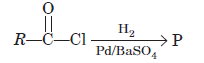
(a) RCHO
(b) RCH3
(c) RCOOH
(d) RCH2OH
Answer
A
Question. An organic compound of molecular formula C3H6O did not give a silver mirror with Tollen’s reagent but gave an oxime with hydroxylamine.
It may be
(a) CH2==CH—CH2—OH
(b) CH3COCH3
(c) CH3CH2CHO
(d) CH2==CH—OCH3
Answer
B
Question. Which of the following compounds will undergo Cannizzaro reaction?
(a) CH3CHO
(b) CH3COCH3
(c) C6H5CHO
(d) C6H5CH2CHO
Answer
C
Nomenclature of Organic Compound
Question. Draw the structure of 3-methylbutanal.
Answer.
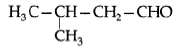
Question. Write the structure of 3-methyl butanal.
Answer.
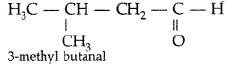
Question. Draw the structural formula of 1-phenyl propan- 1-one molecule.
Answer. 1-phenyl propan-1-one

Question. Write the IUPAC name of the following:
CH3— CH2 — CHO
Answer. IUPAC name : Propan-1-al
Question. Draw the structure of 4-chloropentan-2-one.
Answer.
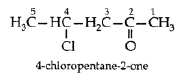
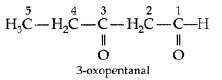
Question. Write the structure of 3-oxopentanal.
Answer.
Question. Write the IUPAC name of the following :
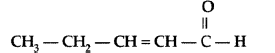
Answer. IUPAC name : Pent-2-enal
Question. Write the structural formula of 1-phenylpentan- 1-one.
Answer.1-Phenylpentan-1-one
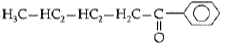
Question. Write the IUPAC name of the following :
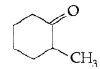
Answer. IUPAC name : 2-methylcyclohexanone
Question. Write the IUPAC name of Ph – CH = CH – CHO.
Answer. IUPAC name : 3-phenylprop-2-enal
Case Based MCQs
Case I : Read the passage given below and answer the following questions :
Carboxylic acids having an a-hydrogen atom when treated with chlorine or bromine in the presence of small amount of red phosphorus gives a-halocarboxylic acids. The reaction is known as Hell-Volhard-Zelinsky reaction.
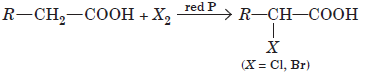
When sodium salt of carboxylic acid is heated with soda lime it loses carbon dioxide and gives hydrocarbon with less number of C-atoms.
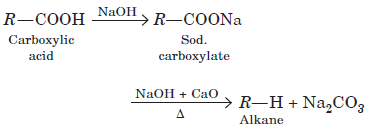
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
Question. Assertion : C6H5COCH2COOH undergoes decarboxylation easily than C6H5COCOOH.
Reason : C6H5COCH2COOH is a b-keto acid.
Answer
A
Question. Assertion : H.V.Z. reaction involves the treatment of carboxylic acids having a-hydrogens with Cl2 or Br2 in presence of small amount of red phosphorus.
Reason : Phosphorus reacts with halogens to form phosphorus trihalides.
Answer
C
Question. Assertion : On heating 3-methylbutanoic acid with soda lime, isobutane is obtained.
Reason : Soda lime is a mixture of NaOH + CaO in the ratio 3 : 1.
Answer
B
Question. Assertion : Propionic acid with Br2/P yields CH2Br—CHBr—COOH.
Reason : Propionic acid has two a-hydrogen atoms.
Answer
D
Question. Assertion : (CH3)3CCOOH does not give H.V.Z reaction.
Reason : (CH3)3CCOOH does not have a-hydrogen atom.
Answer
A
Case II : Read the passage given below and answer the following questions.
Aldehydes and ketones having acetyl group

are oxidised by sodium hypohalate (NaOX) or halogen and alkali (X2 + OH–) to corresponding sodium salt having one carbon atoms less than the carbonyl compound and give a haloform.
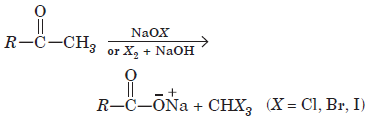
Sodium hypoiodite (NaOI) when treated with compounds containing CH3CO— group gives yellow precipitate of iodoform. Haloform reaction does not affect a carbon-carbon double bond present in the compound.
Question. For the given set of reactions,
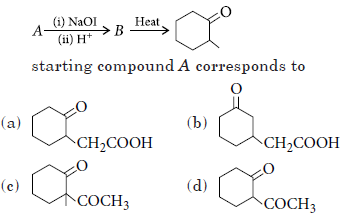
Answer
C
Question. Which of the following compounds is not formed in iodoform reaction of acetone?
(a) CH3COCH2I
(b) ICH2COCH2I
(c) CH3COCHI2
(d) CH3COCI3
Answer
B
Question. An organic compound ‘A’ has the molecular formula C3H6O. It undergoes iodoform test.
When saturated with HCl it gives ‘B’ of molecular formula C9H14O. ‘A’ and ‘B’ respectively are
(a) propanal and mesityl oxide
(b) propanone and mesityl oxide
(c) propanone and 2,6-dimethyl-2,5-heptadien‑4‑one
(d) propanone and propionaldehyde.
Answer
C
Question. Which of the following compounds will give positive iodoform test?
(a) Isopropyl alcohol
(b) Propionaldehyde
(c) Ethylphenyl ketone
(d) Benzyl alcohol
Answer
A
Question. In the following reaction sequence, the correct structures of E, F and G are
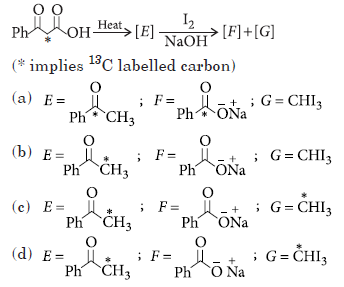
Answer
D
Case III : Read the passage given below and answer the following questions.
The addition reaction of enol or enolate to the carbonyl functional group of aldehyde or ketone is known as aldol addition. The b-hydroxyaldehyde or b-hydroxyketone so obtained undergo dehydration in second step to produce a conjugated enone. The first part of reaction is an addition reaction and the second part is an elimination reaction. Carbonyl compound having a-hydrogen undergoes aldol condensation reaction.

Question. Which of the following compounds would be the main product of an aldol condensation of acetaldehyde and acetone?
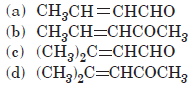
Answer
B
Question. Which of the following does not undergo aldol condensation ?
(a) CH3CHO
(b) CH3CH2CHO
(c) CH3COCH3
(d) C6H5CHO
Answer
D
Question. Which combination of carbonyl compounds gives phenyl vinyl ketone by an aldol condensation?

(a) Acetophenone and Formaldehyde
(b) Acetophenone and acetaldehyde
(c) Benzaldehyde and acetaldehyde
(d) Benzaldehyde and acetone
Answer
A
Question. Condensation reaction is the reverse of which of the following reaction?
(a) Lock and key hypothesis
(b) Oxidation
(c) Hydrolysis
(d) Glycogen formation
Answer
C
Question. Which of the following will undergo aldol condensation?
(a) HCHO
(b) CH3CH2OH
(c) C6H5CHO
(d) CH3CH2CHO
Answer
D
Case IV : Read the passage given below and answer the following questions :
Aldehydes and ketones undergo nucleophilic addition reactions.
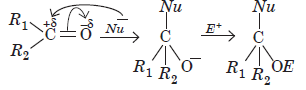
Carbonyl carbon is electron deficient hence acts as an electrophile. Nucleophile attacks on the electrophilic carbon atom of the carbonyl group from a direction perpendicular to the plane of the molecule.
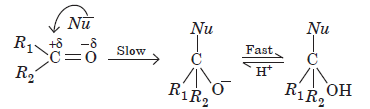
In this process, hybridisation of carbon atom changes from sp2 to sp3 and a tetrahedral alkoxide ion is formed as intermediate. This intermediate captures proton from the reaction medium to give the neutral product. Aldehydes are generally more reactive than ketones in nucleophilic addition reactions.
In the following questions , a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
Question.

Reason : Reactivity of carbonyl group is due to electrophilic nature of carbonyl carbon.
Answer
B
Question. Assertion : (CH3)3CCOC(CH3)3 and acetone can be distinguished by the reaction with NaHSO3.
Reason : HSO3– is the nucleophile in bisulphite addition.
Answer
B
Question. Assertion : Benzaldehyde is more reactive than ethanal towards nucleophilic attack.
Reason : The overall effect of –I and +R effect of phenyl group decreases the electron density on the carbon atom of

group in benzaldehyde.
Answer
A
Question. Assertion : The formation of cyanohydrin from an aldehyde or ketone occurs very slowly with pure HCN. This reaction is catalysed by a base.
Reason : Base generates CN– ion which is a stronger nucleophile.
Answer
A
Question. Assertion : Ease of nucleophilic addition of the compounds

CH3CHO(II) and CH3COCH3(III) is I > II > III.
Reason : Aldehydes and ketones undergo nucleophilic addition reactions.
Answer
D
Case V : Read the passage given below and answer the following questions.
When an aldehyde with no a-hydrogen reacts with concentrated aqueous NaOH, half the aldehyde is converted to carboxylic acid salt and other half is converted to an alcohol. In other words, half of the reactant is oxidized and other half is reduced. This reaction is known as Cannizzaro reaction.
Question. In Cannizzaro reaction given below :

the slowest step is
(a) the attack OH– at the carboxyl group
(b) the transfer of hydride to the carbonyl group
(c) the abstraction of proton from the carboxylic group
(d) the deprotonation of PhCH2OH.
Answer
B
Question. Trichloroacetaldehyde is subjected to Cannizzaro’s reaction by using NaOH. The mixture of the products contains sodium trichloroacetate ion and another compound. The other compounds is
(a) 2, 2, 2-trichloroethanol
(b) trichloromethanol
(c) 2, 2, 2-trichloropropanol
(d) chloroform.
Answer
A
Question. A mixture of benzaldehyde and formaldehyde on heating with aqueous NaOH solution gives
(a) benzyl alcohol and sodium formate
(b) sodium benzoate and methyl alcohol
(c) sodium benzoate and sodium formate
(d) benzyl alcohol and methyl alcohol.
Answer
A
Question. Which of the following reaction will not result in the formation of carbon-carbon bonds?
(a) Cannizzaro reaction
(b) Wurtz reaction
(c) Reimer-Tiemann reaction
(d) Friedel-Crafts’ acylation
Answer
A
Question. Which of the following compounds will undergo Cannizzaro reaction?
(a) CH3CHO
(b) CH3COCH3
(c) C6H5CHO
(d) C6H5CH2CHO
Answer
C
Assertion & Reasoning Based MCQs
For question , a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
Question. Assertion : Boiling point of aldehydes lie in between parent alkanes and corresponding alcohols.
Reason : Aldehydes cannot form intermolecular hydrogen bonds like alcohols.
Answer
B
Question. Assertion : Hydrogen bonding in carboxylic acids is stronger than alcohols.
Reason : Highly branched carboxylic acids are more acidic than unbranched acids.
Answer
C
Question. Assertion : Ketones can be converted into acids by haloform reaction.
Reason : Addition of Grignard reagents to dry ice followed by hydrolysis gives ketones.
Answer
C
Question. Assertion : o-Substituted benzoic acids are generally stronger acids than benzoic acids.
Reason : Increased strength is due to ortho-effect.
Answer
A
Question. Assertion : NaHSO3 is used for the purification of carbonyl compounds.
Reason : They are used in the blending of perfumes and flavouring agents.
Answer
B
Question. Assertion : Acetic acid in vapour state shows a molecular mass of 120.
Reason : It undergoes intermolecular hydrogen bonding.
Answer
A
Question. Assertion : Aromatic aldehydes and formaldehyde undergo Cannizzaro reaction.
Reason : Aromatic aldehydes are almost as reactive as formaldehyde.
Answer
C
Question. Assertion : Nitration of benzoic acid gives m-nitrobenzoic acid.
Reason : Carboxyl group increases the electron density at the meta-position.
Answer
C
Question. Assertion : Carboxylic acids are stabilised by resonance.
Reason : Chloroacetic acid is weaker than acetic acid.
Answer
C
Question. Assertion : Benzaldehyde undergoes aldol condensation.
Reason : Aldehydes having a-hydrogen atom undergo aldol condensation.
Answer
D
Question. Assertion : a-Hydrogen atoms in aldehydes and ketones are acidic.
Reason : The anion left after the removal of a-hydrogen is stabilised by inductive effect.
Answer
C
Question. Assertion : Carboxylic acids have higher boiling points than alkanes.
Reason : Carboxylic acids are resonance hybrids.
Answer
B
Question. Assertion : Formic acid is a stronger acid than benzoic acid.
Reason : pKa of formic acid is lower than that of benzoic acid.
Answer
B
Question. Assertion : m-Chlorobenzoic acid is a stronger acid than p-chlorobenzoic acid.
Reason : In m-chlorobenzoic acid both – I-effect and +R-effect of Cl operate but in p-chlorobenzoic acid only +R-effect of Cl operates.
Answer
C
