Students should refer to Worksheets Class 12 Chemistry Amines Chapter 13 provided below with important questions and answers. These important questions with solutions for Chapter 13 Amines have been prepared by expert teachers for Class 12 Chemistry based on the expected pattern of questions in the Class 12 exams. We have provided Worksheets for Class 12 Chemistry for all chapters on our website. You should carefully learn all the important examinations questions provided below as they will help you to get better marks in your class tests and exams.
Amines Worksheets Class 12 Chemistry
Nomenclature of Organic Compound
Question. Write the structure of prop-2-en-1-amine.
Answer. H2C=CH—H2C—NH2
Question. Write the structure of 2-aminotoluene.
Answer.

Question. Write the IUPAC name of the following compound:
CH3NHCH(CH3)2
Answer. IUPAC name: N-Methylpropan-2-amine
Question. Write the structure of N-methylethanamine.
Answer. Structure of N-methylethanamine : H3C—H2C—NH—CH3
Question. Give the IUPAC name of H2N — CH2—CH2—CH = CH2.
Answer. IUPAC name : But-3-ene-1-amine
Question. Write the IUPAC name of the following compound:
(CH3)2N-CH2CH3
Answer. IUPAC name: N,N-Dimethylethanamine
Question. Write IUPAC name of the following compound :
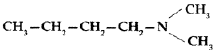
Answer. IUPAC name : N,N-Dimethylbutanamine.
Question. Give IUPAC names of the following compounds :

Answer. (a) IUPAC name : Methylprop-2-en-1-amine (b) IUPAC name : N-Phenyl acetamide.
Question. Write IUPAC name of the following compound:
Answer. N-Ethyl-N-methylethanamine
Question. Write the IUPAC name of the given compound:

Answer. 2, 4, 6-Tribromoaniline
Question. Why is an alkylamine more basic than ammonia?
Answer. Due to electron releasing inductive effect (+I) of alkyl group, the electron density on the nitrogen atom increases and thus, it can donate the lone pair of electrons more easily than ammonia.
Question. Give the IUPAC name of H2N — CH2—CH2—CH = CH2.
Answer. IUPAC name : But-3-ene-1-amine
Question. Write the structure of n-methylethanamine.
Answer.Structure of n-methylethanamine :- H3C—H2C—NH—CH2
Question. Arrange the following compounds in increasing order of solubility in water :
C6H5NH2, (C2H5)2NH, C2H5NH2
Answer. C6H5NH2 < (C2H5)2NH <C2H5NH,
Question. Arrange the following in increasing order of basic strength :
C6H5NH2, C6H5NHCH3, C6H5CH2NH2
Answer. C6H5NH2 < C6H5NHCH3 < C6H5CH2NH2
Question. Give the chemical tests to distinguish between the following pair of compounds : Aniline and Benzylamine
Answer. Distinction between Aniline and Benzylamine : By Nitrous acid test : Benzylamine reacts with HNO2 to form a diazonium salt which being unstable even at low temperature, decomposes with evolution of N2 gas
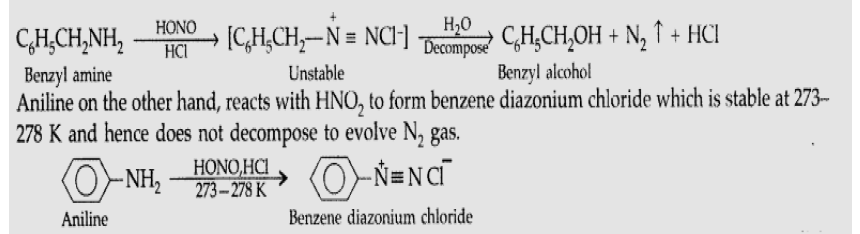
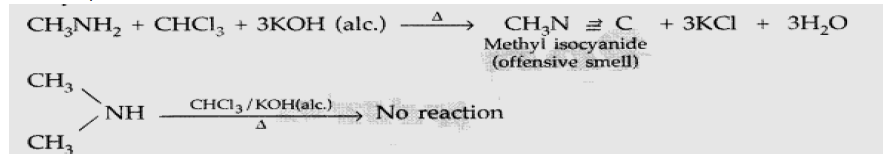
Question. Give the chemical tests to distinguish between the following pair of compounds : Methylamine and Dimethylamine
Answer. Methylamine and Dimethylamine : By Carbylamine test: Methylamine being a primary amine gives this test but Dimethylamine being a secondary amine does not.
Question. Describe the following giving the relevant chemical equation in each case :
(i) Carbylamine reaction
(ii) Hofmann’s bromamide reaction
Answer. (i) Carbylamine reaction : Aliphatic and aromatic primary amines on heating with chloroform and ethanolic KOH form isocyanides or carbylamines which are foul smelling substances. This reaction is known as carbylamines reaction.
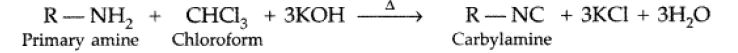
(ii) Hofmann’s bromamide reaction : Primary amines can be prepared by treating an amide with Br2 in an aqueous or alcoholic soln of NaOH.

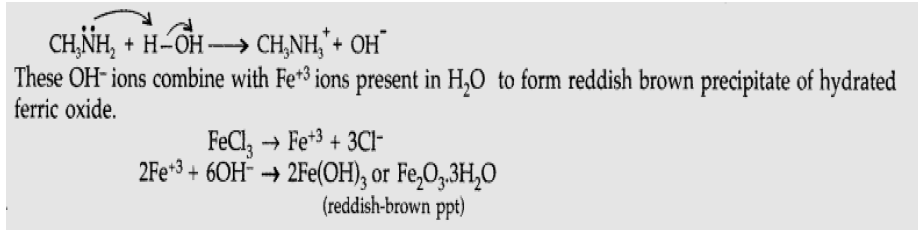
Question. How would you account for the following : (a) Aniline is a weaker base than cyclohexyl amine. (b) Methylamine in aqueous medium gives reddish-brown precipitate with FeCl3 Answer. (a) In aniline, the lone pair of electrons on the N-atom is delocalised over the benzene ring. As a result, the electron density on the nitrogen decreases. But in cyclohexylamine, the lone pair of electrons on N-atom is readily available due to absence of reelections. Hence aniline is weaker base than cyclohexylamine.
(b) Methylamine being more basic than H2O, it accepts a proton from water liberating OH– ions.
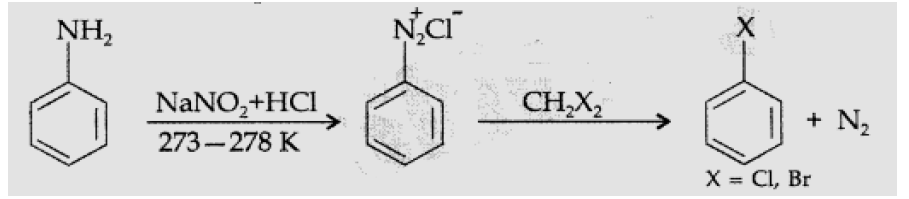
Question. Explain Sandmeyer’s reaction.
Answer. Sandmeyer’s reaction : Aniline reacts with NaNO2 in HCl at 273 – 278 K giving diazonium salt which further reacts with cuprous chloride/bromide to give chloro or bromo benzene.
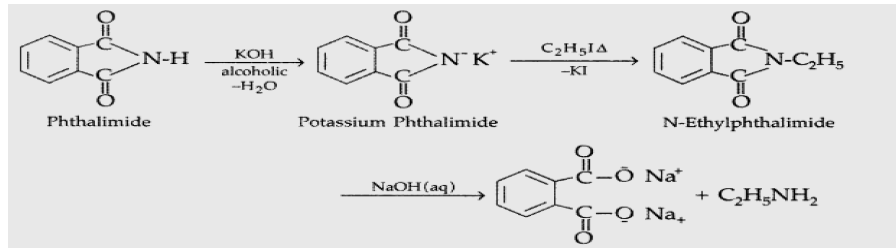
Question. Explain Gabriel Phthalimide reaction.
Answer. Gabriel phthalimide synthesis : It is used to prepare 1° amine (Primary amine). The starting compound is a phthalimide. But aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide. Example :
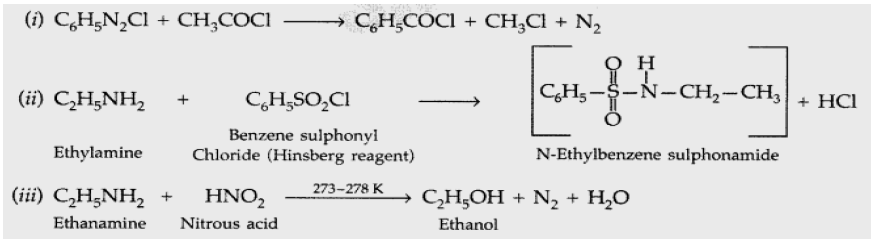
Question. Complete the following reaction equations : (i) C6H5Cl + CH3COCl →
(ii) C2H5NH2 + C6H5SO2Cl →
(iii) C2H5NH2 + HNO2 →
Answer:
Question. In the following cases rearrange the compounds as directed :
(i) In an increasing order of basic strength : C6H5NH2, C6H5 N(CH3)2, (C2H5)2NH and CH3NH2
(ii) In a decreasing order of basic strength : Aniline, p-nitroaniline and p-toluidine
(iii) In an increasing order of pKb values : C2H5NH2, C6H5 NHCH3, (C2H5)2NH and C6H5NH2
Answer: (i) Order of basic strength :
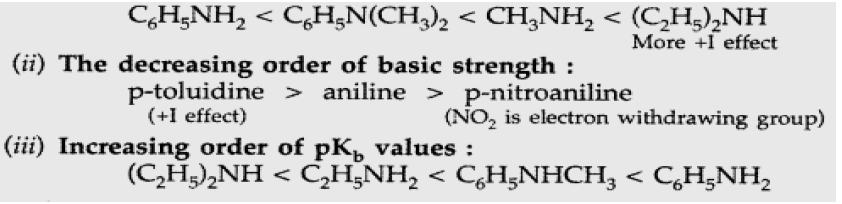
Question. State reasons for the following :
(i) pKb value for aniline is more than that for methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not soluble in water.
(iii) Primary amines have higher boiling points than tertiary amines.
Answer: (i) Higher the pKb value, lower will be the basicity therefore aniline is less basic than methylamine because the lone pair of electrons on nitrogen atom gets delocalized over the benzene ring are unavailable for protonation due to resonance in aniline which is absent in case of alkylamine.
(ii) Ethylamine is soluble in water due to its capability to form H-bonds with water while aniline is insoluble in water due to larger hydrocarbon part which tends to retard the formation of H-bonds.
(iii) Due to presence of two H-atoms on N-atom of primary amines, they undergo extensive intermolecular H-bonding while tertiary amines due to the absence of a H-atom on the N-atom, do not undergo H- bonding. As a result, primary amines have higher boiling points than 3° amines.
Question.Give the structures of A, B and C in the following reactions :
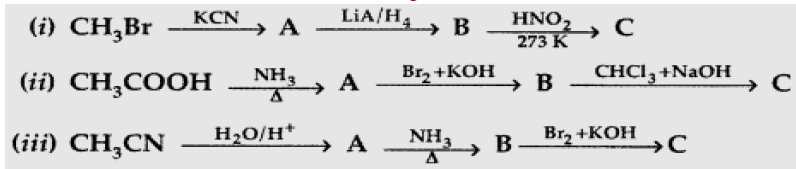
Answer: