Students should refer to Worksheets Class 12 Chemistry Chemical Kinetics Chapter 4 provided below with important questions and answers. These important questions with Chemical Kinetics for Chapter 4 Chemical Kinetics have been prepared by expert teachers for Class 12 Chemistry based on the expected pattern of questions in the Class 12 exams. We have provided Worksheets for Class 12 Chemistry for all chapters on our website. You should carefully learn all the important examinations questions provided below as they will help you to get better marks in your class tests and exams.
Chemical Kinetics Worksheets Class 12 Chemistry
Question. In terms of the ‘Collision Theory of Chemical Kinetics’, the rate of a chemical reaction is proportional to
(a) the change in free energy per second
(b) the change in temperature per second
(c) the number of collisions per second
(d) the number of products molecules
Answer
C
Question. Which one of the following statements for the order of a reaction is incorrect ?
(a) Order can be determined only experimentally.
(b) Order is not influenced by stoichiometric coefficient of the reactants.
(c) Order of reaction is sum of power to the concentration terms of reactants to express the rate of reaction.
(d) Order of reaction is always whole number.
Answer
D
Question. In a slow reaction, rate of reaction generally ………. with time:
(a) decreases
(b) increases
(c) sometimes increases and sometimes decreases.
(d) remains constant
Answer
A
Question. 3A → B + C, it would be a zero order reaction when
(a) the rate of reaction is proportional to square of concentration of A
(b) the rate of reaction remains same at any concentration of A
(c) the rate remains unchanged at any concentration of B and C
(d) the rate of reaction doubles if concentration of B is increased to double
Answer
B
Question. For the reaction 2 A + B → 3C + D
which of the following does not express the reaction rate ?
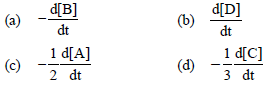
Answer
D
Question. The order of a reaction with rate equal to k[A]3/2 [B]–1/2 is :
(a) 1
(b) -1/2
(c) -3/2
(d) 2
Answer
A
Question. Which of the following reaction does not occur fastly ?
(a) Precipitation of AgCl by mixing aqueous solutions of AgNO3 and NaCl.
(b) Burning of gasoline
(c) Rusting of iron
(d) Burning of LPG for cooking
Answer
C
Question. Chemical kinetics is a study to find out
(a) the feasibility of a chemical reaction
(b) extent to which a reaction will proceed
(c) speed of a reaction
(d) All of the above
Answer
C
Question. Rate of a reaction can be defined as
(a) the rate of decrease in concentration of any one of the reactants
(b) the rate of increase in concentration of any one of the products
(c) the rate of decrease in concentration of any one of the reactants or the rate of increase in concentration of any one of the products
(d) the sum of rate of decrease in concentration of all the reactants or the rate of increase in concentration of all the products
Answer
C
Question. The rate of reaction
(a) increases as the reaction proceeds
(b) decreases as the reaction proceeds
(c) remains the same as the reaction proceeds
(d) may decrease or increase as the reaction proceeds
Answer
D
Question. The unit of rate of reaction is
(a) mole/dm3
(b) mole/pound
(c) mole/dm3 sec
(d) mole/cm3
Answer
C
Question.In the rate equation, when the conc. of reactants is unity then rate is equal to
(a) specific rate constant
(b) average rate constant
(c) instantaneous rate constant
(d) None of above
Answer
A
Question. Collision theory is applicable to
(a) first order reactions
(b) zero order reactions
(c) bimolecular reactions
(d) intra-molecular reactions
Answer
C
Question. The rate of reaction between two specific time intervals is called
(a) instantaneous rate
(b) average rate
(c) specific rate
(d) ordinary rate
Answer
B
Question. Instantaneous rate of a chemical reaction is
(a) rate of reaction in the beginning
(b) rate of reaction at the end
(c) rate of reaction at a given instant
(d) rate of reaction between two specific time intervals
Answer
C
Question. At the beginning the decrease in the conc. of reactants is
(a) slow
(b) moderate
(c) rapid
(d) None of above
Answer
C
Question. For the following reaction: NO2(g) + CO(g) → NO(g) + CO2(g), the rate law is: Rate = k [NO2]2. If 0.1 mole of gaseous carbon monoxide is added at constant temperature to the reaction mixture which of the following statements is true?
(a) Both k and the reaction rate remain the same
(b) Both k and the reaction rate increase
(c) Both k and the reaction rate decrease
(d) Only k increases, the reaction rate remain the same
Answer
A
Question. In a reaction A → Products, when start is made from 8.0 × 10–2 M of A, half-life is found to be 120 minute. For the initial concentration 4.0 × 10–2 M, the half-life of the reaction
becomes 240 minute. The order of the reaction is :
(a) zero
(b) one
(c) two
(d) 0.5
Answer
C
Question. The rate of the reaction 2NO+ Cl2 → 2NOCl is given by the rate equation rate = k [NO]2 [Cl2]
The value of the rate constant can be increased by:
(a) increasing the concentration of NO.
(b) increasing the temperature.
(c) increasing the concentration of the Cl2
(d) doing all of the above
Answer
B
Question. What is order with respect to A, B, C, respectively
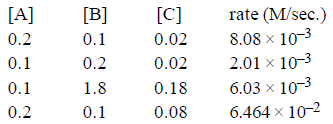
(a) –1, 1, 3/2
(b) –1, 1, 1/2
(c) 1, 3/2, –1
(d) 1, –1, 3/2
Answer
D
Question. Order of reaction can be
(a) 0
(b) fraction
(c) whole number
(d) integer, fraction, zero
Answer
D
Question. Units of rate constant of first and zero order reactions in terms of molarity M unit are respectively
(a) sec–1, Msec–1
(b) sec–1, M
(c) Msec–1, sec–1
(d) M, sec–1.
Answer
A
Question. A reaction involving two different reactants can never be
(a) bimolecular reaction
(b) second order reaction
(c) first order reaction
(d) unimolecular reaction
Answer
D
Question. In the Arrhenius plot of ln k vs 1/T, a linear plot is obtained with a slope of –2 × 104 K. The energy of activation of the reaction (in kJ mole–1) is (R value is 8.3 J K–1 mol–1)
(a) 83
(b) 166
(c) 249
(d) 332
Answer
B
Question. For the following homogeneous reaction,
A + B k→ C
the unit of rate constant is
(a) sec–1
(b) sec–1 mol L–1
(c) sec–1 mol–1 L
(d) sec–1 mol–2 L2
Answer
C
Question. The rate constant for the reaction
2N2O5 → 4NO2 + O2 is 3.10 × 10–5 sec–1. If the rate is 2.4 × 10–5 mol litre–1 sec–1 then the concentration of N2O5 (in mol litre–1) is :
(a) 0.04
(b) 0.8
(c) 0.07
(d) 1.4
Answer
B
Question. A zero order reaction is one whose rate is independent of
(a) the concentration of the reactants
(b) the temperature of reaction
(c) the concentration of the product
(d) the material of the vessel in which reaction is carried out
Answer
A
Question. The slope in Arrhenius plot, is equal to:
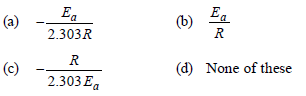
Answer
A
Question. The activation energy for a reaction is 9.0 kcal/mol. The increase in the rate constant when its temperature is increased from 298K to 308K is
(a) 63%
(b) 50%
(c) 100%
(d) 10%
Answer
A
Question. In the reaction 2A + B → A2B, if the concentration of A is doubled and that of B is halved, then the rate of the reaction will:
(a) increase 2 times
(b) increase 4 times
(c) decrease 2 times
(d) remain the same
Answer
A
Question. In the Haber process for the manufacture of ammonia the following catalyst is used
(a) Platinized asbestos
(b) Iron with molybdenum as promoter
(c) Copper oxide
(d) Alumina
Answer
B
Question. What is the activation energy for a reaction if its rate doubles when the temperature is raised from 20°C to 35°C? (R = 8.314 J mol–1 K–1)
(a) 269 kJ mol–1
(b) 34.7 kJ mol–1
(c) 15.1 kJ mol–1
(d) 342 kJ mol–1
Answer
B
Question. If the rate of a gaseous reaction is independent of pressure, the order of reaction is:
(a) 0
(b) 1
(c) 2
(d) 3
Answer
A
Question. Point out the wrong statement:
For a first order reaction
(a) time for half-change (t1/2) is independent of initial concentration
(b) change in the concentration unit does not change the rate constant (k)
(c) time for half-change × rate constant = 0.693
(d) the unit of k is mole–1 min–1
Answer
D
Question. If the rate of the reaction is equal to the rate constant, the order of the reaction is
(a) 3
(b) 0
(c) 1
(d) 2
Answer
B
Question. The rate of reaction is doubled for every 10°C rise in temperature. The increase in reaction rate as a result of temperature rise from 10°C to 100°C is
(a) 112
(b) 512
(c) 400
(d) 614
Answer
B
Question. In a reaction, when the concentration of reactant is increased two times, the increase in rate of reaction was four times.
Order of reaction is :
(a) zero
(b) 1
(c) 2
(d) 3
Answer
C
Question. For the reaction A + 2B → C, rate is given by R = [A] [B]2 then the order of the reaction is
(a) 3
(b) 6
(c) 5
(d) 7
Answer
B
Question. The rate law for the single- step reaction 2A+ B→2C, is given by:
(a) rate = k [A].[B]
(b) rate = k [A]2.[B]
(c) rate = k [2A].[B]
(d) rate = k [A]2.[B]o
Answer
B
Question. Half life of a first order reaction is 4s and the initial concentration of the reactant is 0.12 M. The concentration of the reactant left after 16 s is
(a) 0.0075 M
(b) 0.06 M
(c) 0.03 M
(d) 0.015 M
Answer
A
Question. Which one of the following reactions is a true first order reaction?
(a) Alkaline hydrolysis of ethyl acetate
(b) Acid catalyst hydrolysis of ethyl acetate
(c) Decomposition of N2O
(d) Decomposition of gaseous ammonia on a hot platinum surface
Answer
C
Question. The term – dc/dt in a rate equation refers to :
(a) the conc. of a reactant
(b) the decrease in conc. of the reactant with time
(c) the velocity constant of reaction
(d) None of these
Answer
B
Question. For a reaction A + B → C + 2D, experimental results were collected for three trials and the data obtained are given below:
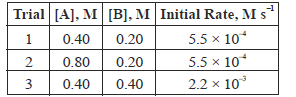
The correct rate law of the reaction is
(a) rate = k[A]0 [B]2
(b) rate = k[A] [B]2
(c) rate = k[A] [B]
(d) rate = k[A] [B]0
Answer
A
Question. The rate law for the reaction
xA + yB → mP + nQ is Rate = k [A]c[B]d. What is the total order of the reaction?
(a) (x + y)
(b) (m + n)
(c) (c + d)
(d) x/y
Answer
C
Question. For the reaction 2 A + B → 3C + D
which of the following does not express the reaction rate ?
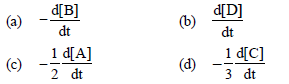
Answer
D
Question. Select the rate law that corresponds to the data shown for the following reaction :
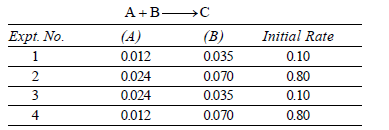
(a) Rate = k[B]3
(b) Rate = k [B]4
(c) Rate = k [A] [B]3
(d) Rate = k [A]2 [B]2
Answer
A
Question. Rate of which reaction increases with temperature :
(a) of any type of reactions
(b) of exothemic reactions
(c) of endothemic reactions
(d) of none
Answer
A
Question. For the reaction,
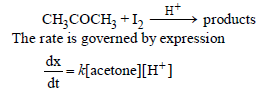
The order w.r.t. I2 is:
(a) 1
(b) 0
(c) 3
(d) 2
Answer
B
Question. The rate constant of a reaction is 3.00 × 103 L mol–1 sec–1.The order of this reaction will be:
(a) 0
(b) 1
(c) 2
(d) 3
Answer
C
Question. The chemical reaction 2O3 →3O2 proceeds as follows:
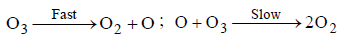
the rate law expression should be
(a) r = k[O3]2
(b) r = k [O3]2[O2]–1
(c) r = k3 [O3][O2]2 ,
(d) r = [O3][O2]2
Answer
B
Question. According to the collision theory of reaction rates, the rate of reaction increases with temperature due to
(a) greater number of collision
(b) higher velocity of reacting molecules
(c) greater number of molecules having the activation energy
(d) decrease in the activation energy
Answer
A
Question. The average rate and instantaneous rate of a reaction are equal
(a) at the start
(b) at the end
(c) in the middle
(d) when two rate have time interval equal to zero
Answer
D
Question. The rate of reaction depends upon the
(a) volume
(b) force
(c) pressure
(d) conc. of reactants
Answer
D
Question. The plot that represents the zero order reaction is :

Answer
C
Question. In respect of the equation k = Ae-Ea / RT in chemical kinetics, which one of the following statements is correct ?
(a) A is adsorption factor
(b) Ea is energy of activation
(c) R is Rydberg’s constant
(d) k is equilibrium constant
Answer
B
Question. In Arrhenius plot, intercept is equal to
(a) -Ea/R
(b) ln A
(c) ln k
(b) log10a
Answer
B
Question. The plot of concentration of the reactant vs time for a reaction is a straight line with a negative slope. The reaction follows a rate equation
(a) zero order
(b) first order
(c) second order
(d) third order
Answer
A
Question. The half-life of a reaction is inversely proportional to the square of the initial concentration of the reactant. Then the order of the reaction is
(a) 0
(b) 1
(c) 2
(d) 3
Answer
D
Question. The rate equation for a reaction,
N2O → N2 + 1/2O2
is Rate = k[N2O]0 = k. If the initial concentration of the reactant is a mol Lit–1, the half-life period of the reaction is
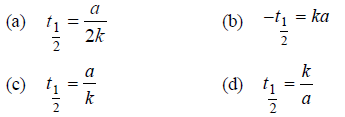
Answer
A
Question. The rate of a chemical reaction tells us about,
(a) the reactants taking part in reaction
(b) the products formed in the reaction
(c) how slow or fast the reaction is taking place
(d) None of the above
Answer
C
Question. The reaction A→ B follows first order kinetics. The time taken for 0.8 mole of A to produce 0.6 mole of B is 1 hour.What is the time taken for conversion of 0.9 mole of A to produce 0.675 mole of B?
(a) 2 hours
(b) 1 hour
(c) 0.5 hour
(d) 0.25 hour
Answer
B
Question. The rate of a first order reaction is 1.5 × 10–2 mol L–1 min–1 at 0.5 M concentration of the reactant. The half life of the reaction is
(a) 0.383 min
(b) 23.1 min
(c) 8.73 min
(d) 7.53 min
Answer
B
Question. The rate law for a reaction between the substances A and B
is given by Rate = k [A]n [B]m
On doubling the concentration of A and halving the concentration of B, the ratio of the new rate to the earlier rate of the reaction will be as
(a) (m + n)
(b) (n – m)
(c) 2(n – m)
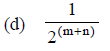
Answer
C
Question. The rate constant of a first order reaction is 6.9×10-3s-1 .How much time will it take to reduce the initial concentration to its 1/8th value?
(a) 100 s
(b) 200 s
(c) 300 s
(d) 400 s
Answer
C
Question. A catalyst
(a) increases the rate of reaction by decreasing ΔG of a reaction.
(b) increases the rate of reaction by increasing ΔG of a reaction.
(c) increases the rate of reaction by decreasing activation energy of the forward reaction.
(d) increases the rate of reaction by providing an alternative pathway via an intermediate with lower activation energy.
Answer
D
Question. t 1/4 can be taken as the time taken for the concentration of a reactant to drop to 3/4 of its initial value. If the rate constant for a first order reaction is k, the t 1/4 can be written as
(a) 0.75/k
(b) 0.69/k
(c) 0.29/k
(d) 0.10/k
Answer
C
Question. Which of the following has been used to explain the subject of chemical kinetics
(a) Collision theory of bimolecular reactions
(b) The activated complex theory
(c) Arrhenius equation
(d) All of these
Answer
D
Question. In a first-order reaction A → B, if k is rate constant and inital concentration of the reactant A is 0.5 M, then the halflife is
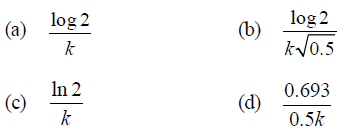
Answer
B
Question. Consider the reaction, 2A + B → products. When concentration of B alone was doubled, the half-life did not change. When the concentration of A alone was doubled,the rate increased by two times. The unit of rate constant for this reaction is
(a) s–1
(b) L mol–1 s–1
(c) no unit
(d) mol L–1 s–1.
Answer
B
Question. Order of reaction is decided by
(a) temperature
(b) mechanism of reaction as well as relative concentration of reactants
(c) molecularity
(d) pressure
Answer
B
Question. Velocity constant k of a reaction is affected by
(a) change in the concentration of the reactant
(b) change of temperature
(c) change in the concentration of the product
(d) None of the above
Answer
B
Question. The decomposition of N2O5 occurs as 2N2O5 → 4NO2 + O2 and follows Ist order kinetics, hence:
(a) the reaction is unimolecular
(b) the reaction is bimolecular
(c) t1/2 ∝ a0
(d) None of these
Answer
C
Question. In a first-order reaction A → B, if k is rate constant and inital concentration of the reactant A is 0.5 M, then the halflife is
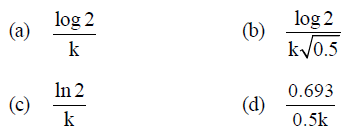
Answer
C
Question. For a first order reaction, a plot of log (a – x) against time is a straight line with a negative slope equal to
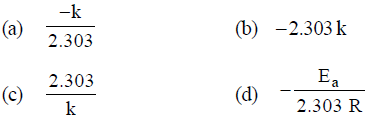
Answer
A
Question. The value of rate constant of a pseudo first order reaction _________.
(a) depends on the concentration of reactants present in small amount.
(b) depends on the concentration of reactants present in excess.
(c) is independent on the concentration of reactants.
(d) depends only on temperature.
Answer
B
Question. Consider the reaction
N2 (g) + 3H2 (g) → 2 NH3 (g)
The equality relationship between
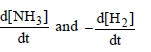
is


Answer
A
Question. A reaction having equal energies of activation for forward and reverse reaction has :
(a) ΔG = 0
(b) ΔH = 0
(c) ΔH = ΔG = ΔS = 0
(d) ΔS = 0
Answer
B
Question. The order of a reaction, with respect to one of the reacting component Y, is zero. It implies that:
(a) the reaction is going on at a constant rate
(b) the rate of reaction does not vary with temperature
(c) the reaction rate is independent of the concentration of Y
(d) the rate of formation of the activated complex is zero
Answer
C
Question. Plots showing the variation of the rate constant (k) with temperature (T) are given below. The plot that follows Arrhenius equation is

(a) 410 kJ mol–1
(b) –110 kJ mol–1
(c) 110 kJ mol–1
(d) – 410 kJ mol–1
Answer
A
Question. Activation energy of a chemical reaction can be determined by
(a) evaluating rate constant at standard temperature
(b) evaluating velocities of reaction at two different temperatures
(c) evaluating rate constants at two different temperatures
(d) changing concentration of reactants
Answer
C
Question. A reaction proceeds by first order, 75% of this reaction was completed in 32 min. The time required for 50% completion is
(a) 8 min
(b) 16 min
(c) 20 min
(d) 24 min
Answer
B
Question. Rate of a reaction can be expressed by Arrhenius equation as : k = Ae-Ea / RT In this equation, Ea represents
(a) the total energy of the reacting molecules at a temperature, T
(b) the fraction of molecules with energy greater than the activation energy of the reaction
(c) the energy below which all the colliding molecules will react
(d) the energy below which colliding molecules will not react
Answer
D
Question. Nitrogen monoxide, NO, reacts with hydrogen, H2, according to the following equation:
2NO(g) + 2H2(g) → N2(g) + 2H2O(g)
If the mechanism for this reaction were,
2NO(g) + H2(g) → N2(g) + H2O2(g) ; slow
H2O2(g) + H2(g) → 2H2O(g) ; fast
Which of the following rate laws would we expect to obtain experimentally?
(a) Rate = k[H2O2][H2]
(b) Rate = k[NO]2[H2]
(c) Rate = k[NO]2[H2]2
(d) Rate = k[NO][H2]
Answer
C
Question. The minimum energy required for the reacting molecules to undergo reaction is :
(a) potential energy
(b) kinetic energy
(c) thermal energy
(d) activation energy
Answer
D
Question. The rate constant for a first order reaction whose half-life, is 480 seconds is :
(a) 2.88 × 10–3 sec–1
(b) 2.72 × 10–3 sec–1
(c) 1.44 × 10 –3 sec–1
(d) 1.44 sec–1
Answer
C
Question. The reason for almost doubling the rate of reaction on increasing the temperature of the reaction system by 10°C is
(a) the value of threshold energy increases
(b) collision frequency increases
(c) the fraction of the molecule having energy equal to threshold energy or more increases
(d) activation energy decreases
Answer
C
Question. In a reversible reaction the energy of activation of the forward reaction is 50 kcal. The energy of activation for the reverse reaction will be
(a) < 50 kcal
(b) either greater than or less than 50 kcal
(c) 50 kcal
(d) > 50 kcal
Answer
B
Question.The unit of rate constant for a zero order reaction is
(a) mol L–1 s–1
(b) L mol–1 s–1
(c) L2 mol–2 s–1
(d) s–1
Answer
A
Question. Which of the following statements best describes how a catalyst works?
a) A catalyst changes the potential energies of the reactants and products.
(b) A catalyst decreases the temperature of the reaction which leads to a faster rate.
(c) A catalyst lowers the activation energy for the reaction by providing a different reaction mechanism.
(d) A catalyst destroys some of the reactants, which lowers the concentration of the reactants.
Answer
C
Question. According to collision theory, which of the following is NOT a true statement concerning a catalyst?
(a) A catalyst changes the temperature of reaction.
(b) The mechanism of a reaction will change when a catalyst is added.
(c) A catalyst provides a different activation energy for a reaction.
(d) A catalyst changes the speed of a reaction, but not the equilibrium constant.
Answer
A
Question. Which of the following influences the rate of a chemical reaction performed in solution?
(a) Temperature
(b) Activation energy
(c) Presence of a catalyst
(d) All of the above influence the rate
Answer
D
Question. How can be activation energy for a reaction be determined graphically?
(a) Plot k versus T, the slope of the line will be equal to Ea
(b) Plot 1/[A]t versus t, the slope of the line will be equal to Ea
(c) Plot ln [A]t versus t, the slope of the line will be equal to – Ea
(d) Plot ln k versus 1/T, the slope of the line will be equal to – Ea / R
Answer
D
Question. Which of the following is not a first order reaction ?
(a) Hydrogenation of ethene
(b) Natural radioactive decay of unstable nuclei
(c) Decomposition of HI on gold surface
(d) Decomposition of N2O
Answer
C
Question. The Arrhenius equation expressing the effect of temperature on the rate constant of the reaction is
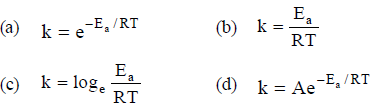
Answer
D
Question. In an exothermic reaction if ΔH is the enthalpy then activation energy is
(a) more than ΔH
(b) less than ΔH
(c) equal to ΔH
(d) none of the above
Answer
D
Question. A chemical reaction was carried out at 300 K and 280 K. The rate constants were found to be k1 and k2 respectively. then
(a) k1 = 4k1
(b) k2 = 2k1
(c) k2 = 0.25 k1
(d) k2 = 0.5 k1
Answer
C
Question. For a first order reaction, the plot of log K against 1/T is a straight line. The slope of the line is equal to
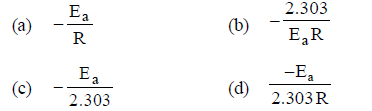
Answer
D
Question. During the kinetic study of the reaction, 2A + B → C + D,following results were obtained:
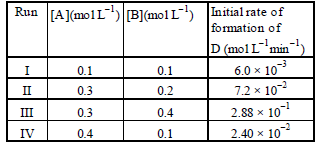
Based on the above data which one of the following is correct?
(a) rate = k [A]2 [B]
(b) rate = k[A] [B]
(c) rate = k[A]2[B]2
(d) rate = k[A] [B]2
Answer
D
Question. For the reaction H2(g) + Br2 (g) → 2HBr (g), the experimental data suggest, rate = k[H2][Br2]1/2. The molecularity and order of the reaction are respectively
(a) 2, 3/2
(b)3/2,3/2
(c) 1, 1
(d) 1, 1/2
Answer
A
MATCHING TYPE QUESTIONS
Question. Match the columns
Column-I Column-II
(A) The decomposition (p) Zero order reaction
of gaseous ammonia
on a hot platinum
surface
(B) The thermal (q) Pseudo first order reaction.
decomposition of HI
on gold surface
(C) All natural and (r) Zero order reaction at high pressure
artificial radioactive
decay of unstable
nuclei
(D) Inversion of cane sugar (s) First order reaction.
(a) A – (r), B – (p), C – (s), D – (q)
(b) A – (r), B – (s), C – (q), D – (p)
(c) A – (q), B – (s), C – (p), D – (r)
(d) A – (q), B – (p), C – (s), D – (p)
Answer
A
Question. Match the columns
Column-1 Column-II
(A) Zero order reaction (p) L mole–1 sec–1
(B) First order reaction (q) mole L–1 sec–1
(C) Second order reaction (r) sec–1
(a) A – (q), B – (r) ,C – (p)
(b) A – (q), B – (p) ,C – (r)
(c) A – (p), B – (q) ,C – (r)
(d) A – (p), B – (r) ,C – (q)
Answer
B
Question. Consider the energy diagram of a reaction : B → A, on the basis of given diagram select the correct code for matching Column-I and Column-II.
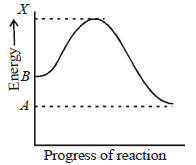
Column-I Column-II
(A) X – A (p) Enthalpy of reaction
(B) X – B (q) Energy of transition state
(C) A – B (r) Activation energy of forward reaction
(D) X (s) Activation energy of backward reaction
(a) A – (s), B – (r), C – (q), D – (p)
(b) A – (q), B – (r), C – (p), D – (s)
(c) A – (r), B – (s), C – (p), D – (q)
(d) A – (s), B – (r), C – (p), D – (q)
Answer
D
Question. Match the columns.
Column-I Column-II
(A) Catalyst alters the rate (p) cannot be fraction or zero
of reaction
(B) Molecularity (q) proper orientation is not there always.
(C) Second half life of first (r) by lowering the activation enrgy
order reaction
(D) Energetically favourable (s) is same as the first
reactions are sometimes
slow
(a) A – (q), B – (r), C – (s), D – (p)
(b) A – (r), B – (s), C – (p), D – (q)
(c) A – (r), B – (p), C – (s), D – (q)
(d) A – (p), B – (r), C – (s), D – (q)
Answer
C
Question. Match the columns
Column – I Column – II
(A) Number of collisions per (p) Effective collisions.
second per unit volume
of the reaction mixture.
(B) Fraction of molecules (q) Collision frequency
with energies equal to
or greater than Ea
(C) Molecules for which (r) e-Ea /RT
Rate = ZABe-Ea /RT
shows significant deviations
(D) Collision in which molecules (s) Complex molecules
collide with sufficient K.E.
and proper orientation.
(a) A – (q), B – (r), C – (s), D – (p)
(b) A – (r), B – (q), C – (s), D – (p)
(c) A – (q), B – (s), C – (r), D – (p)
(d) A – (q), B – (r), C – (p), D – (s)
Answer
A
Question. Match the columns
Column-I Column-II
(A) Mathematical expression for rate (p) rate constant
of reaction
(B) Rate of reaction for zero order (q) rate law
reaction is equal to
(C) Units of rate constant for zero (r) order of slowest
order reaction is same as that of step
(D) Order of a complex reaction is (s) rate of reaction
determined by
(a) A – (q), B – (p), C – (s), D – (r)
(b) A – (r), B – (p), C – (s), D – (q)
(c) A – (q), B – (s), C – (p), D – (r)
(d) A – (p), B – (q), C – (s), D – (r)
Answer
A
Question. Match the columns
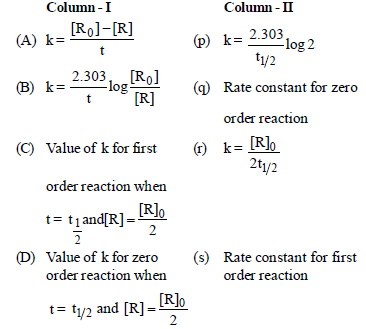
(a) A – (s), B – (q), C – (p), D – (r)
(b) A – (q), B – (s), C – (p), D – (r)
(c) A – (q), B – (p), C – (s), D – (r)
(d) A – (q), B – (s), C – (p), D – (t)
Answer
B
CRITICAL THINKING TYPE QUESTIONS
Question. The bromination of acetone that occurs in acid solution is represented by this equation.
CH3COCH3 (aq) + Br2 (aq) → CH3COCH2Br (aq) + H+ (aq)+ Br– (aq)
These kinetic data were obtained for given reaction concentrations.
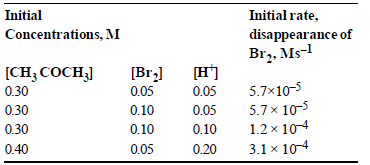
Based on given data, the rate equations is:
(a) Rate = k[CH3COCH3][H+]
(b) Rate = k [CH3COCH3][Br2]
(c) Rate = k [CH3COCH3] [Br2] [H+]2
(d) Rate = k [CH3COCH3][Br2] [H+]
Answer
A
Question. The rate of the reaction 2N2O5 → 4NO2 + O2 can be written in three ways :
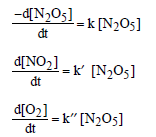
The relationship between k and k’ and between k and k” are:
(a) k’ = 2k ; k’ = k
(b) k’ = 2k ; k” = k / 2
(c) k’ = 2k ; k” = 2k
(d) k’ = k ; k” = k
Answer
D
Question. The reaction of hydrogen and iodine monochloride is given as:
H2 (g) + 2ICl(g) → 2HCl(g) + I2 (g)
The reaction is of first order with respect to H2(g) and ICI(g),
following mechanisms were proposed.
Mechanism A:
H2 (g) + 2ICl(g) → 2HCl(g) + I2 (g)
Mechanism B:
H2 (g) + ICl(g) → HI(g);slow
HI(g) + ICl(g) → HCl(g) + I2 (g);fast
Which of the above mechanism(s) can be consistent with the given information about the reaction?
(a) A and B both
(b) Neither A nor B
(c) A only
(d) B only
Answer
D
Question. A graph plotted between log k vs 1/T for calculating activation energy is shown by
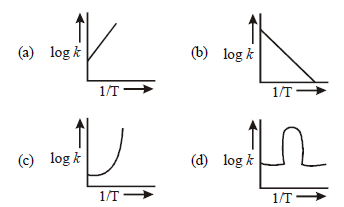
Answer
B
Question. The hypothetical reaction A2 + B2 → 2AB ; follows the following mechanism

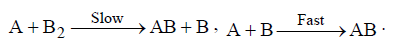
The order of the overall reaction is
(a) 0
(b) 1
(c) 2
(d) 3/2
Answer
D
Question. For the first order reaction
C2H4O(g) → CH4 (g) + CO(g) ,the initial pressure of
C2H4O(g) is 80 torr and total pressure at the end of 20 minutes is 120 torr. The time needed for 75% decomposition of C2H4O would be :
(a) 20 minutes
(b) 40 minutes
(c) 80 minutes
(d) 120 minutes
Answer
B
Question. The initial rates of reaction
3A + 2B + C → Products, at different initial
concentrations are given below:
Initial rate, [A]0, M [B]0, M [C]0, M
Ms–1
5.0 × 10–3 0.010 0.005 0.010
5.0 × 10–3 0.010 0.005 0.015
1.0 × 10–2 0.010 0.010 0.010
1.25 × 10–3 0.005 0.005 0.010
The order with respect to the reactants, A, B and C are respectively
(a) 3, 2, 0
(b) 3, 2, 1
(c) 2, 2, 0
(d) 2, 1, 0
Answer
D
Question. In the following reaction, how is the rate of appearance of underlined product related to the rate of disappearance of the underlined reactant ?
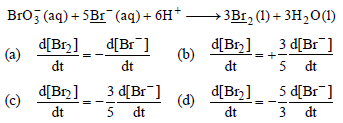
Answer
C
Question. Consider a reaction aG + bH → Products. When concentration of both the reactants G and H is doubled, the rate increases by eight times. However, when concentration of G is doubled keeping the concentration of H fixed, the rate is doubled. The overall order of the reaction is
(a) 0
(b) 1
(c) 2
(d) 3
Answer
D
Question. Consider the following reaction at 25°C:
(CH3)3COH(l) + HCl(aq) → (CH3)3CCl(l) + H2O(l)
The experimentally determined rate law for this reaction indicates that the reaction is of first order in (CH3)3COH and that the reaction is of first order overall. Which of the following would produce an increase in the rate of this reaction?
(a) Increasing the concentration of (CH3)3COH
(b) Increasing the concentration of HCl
(c) Decreasing the concentration of HCl
(d) Decreasing the concentration of (CH3)3CCl
Answer
A
Question. Which of the following statements is incorrect ?
(a) Energy is always released when activated complex decomposes to form products.
(b) Peak of the energy distribution curve corresponds to the most probable potential energy.
(c) Peak of the energy distribution curve corresponds to the most probable kinectic energy.
(d) When the temperature is raised maximum of energy distribution curve moves to higher energy value and broadens out.
Answer
B
Question. The following data pertains to reaction between A and B :
S.N o. [A] mol L–1 [B] mol L–1 Rate (mol L–1 time–1)
1 1.0 × 10–2 2.0 × 10–2 2.0 × 10–4
2 2.0 × 10–2 2.0 × 10–2 4.0 × 10–4
3 2.0 × 10–2 4.0 × 10–2 8.0 × 10–4
Which of the following inference(s) can be drawn from the above data ?
(i) Rate constant of the reaction is 1.0 × 10–4.
(ii) Rate law of the reaction is : rate = k[A][B]
(iii) Rate of reaction increases four times on doubling the concentration of both the reactants.
Select the correct answer using the codes given below :
(a) (i), (ii) and (iii)
(b) (i) and (ii)
(c) (ii) and (iii)
(d) (iii) only
Answer
C
Question. Collision theory is used to explain how chemical species undergo a reaction. Using this theory and the kinetic molecular model, which of the following does NOT influence the rate of a chemcial reaction?
(a) The temperature of the system
(b) The geometry or orientation of the collision
(c) The velocity of the reactants at the point of collision
(d) All of the above influence the rate
Answer
D
Question. A reactant (A) froms two products :
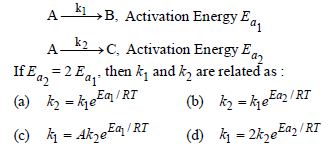
Answer
C
Question. If half-life of a substance is 5 yrs, then the total amount of substance left after 15 years, when initial amount is 64 grams is
(a) 16 grams
(b) 2 grams
(c) 32 grams
(d) 8 grams.
Answer
D
Question. The integrated rate equations can be determined for
(a) zero order reactions
(b) first order reactions
(c) second order reactions
(d) Both (a) and (b)
Answer
D
Question. In a 1st order reaction, reactant concentration C varies with time t as :
(a) 1/C increases linearly with t
(b) log C decreases linearly with t
(c) C decreases with 1/t
(d) log C decreases with 1/t
Answer
B
Question. During decomposition of an activated complex.
(i) energy is always released
(ii) energy is always absorbed
(iii) energy does not change
(iv) reactants may be formed
(a) (i), (ii) and (iii)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii), (iii) and (iv)
Answer
B
Question. Which of the following graph(s) is/are correct for a zero order reaction?

(a) (ii) and (iii)
(b) (i), (ii) and (iii)
(c) (ii), (iii) and (iv)
(d) (i) and (iv)
Answer
D
Question. Diazonium salt decomposes as
C6H5N2+Cl– + C6H5Cl + N2
At 0°C, the evolution of N2 becomes two times faster when the initial concentration of the salt is doubled. Therefore, it is
(a) a first order reaction
(b) a second order reaction
(c) independent of the initial concentration of the salt
(d) a zero order reaction
Answer
A
Question. For a first order reaction A → P, the temperature (T) dependent rate constant (k) was found to follow the equation log k = – (2000) 1/T + 6.0 . The pre-exponential factor
A and the activation energy Ea, respectively, are
(a) 1.0 × 106 s–1 and 9.2 kJ mol–1
(b) 6.0 s–1 and 16.6 kJ mol–1
(c) 1.0 × 106 s–1 and 16.6 kJ mol–1
(d) 1.0 × 106 s–1 and 38.3 kJ mol–1
Answer
D
161. Which of the following statements is not correct for the catalyst?
(a) It catalyses the forward and backward reaction to the same extent.
(b) It alters ΔG of the reaction.
(c) It is a substance that does not change the equilibrium constant of a reaction.
(d) It provides an alternate mechanism by reducing activation energy between reactants and products.
Answer
B
Question. The activation energies of two reactions are E1 and E2 (E1 > E2). If the temperature of the system is increased from T1 to T2, the rate constant of the reactions changes from k1 to k1‘ in the first reaction and k2 to k2‘ in the second reaction. Predict which of the following expression is correct?
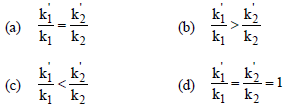
Answer
D
Question. The decomposition of ammonia on tungsten surface at 500 K follows zero order kinetics. The half-life period of this reaction is 45 minutes when the initial pressure is 4 bar. The half-life period (minutes) of the reaction when the initial pressure is 16 bar at the same temperature is
(a) 120
(b) 60
(c) 240
(d) 180
Answer
D
Question. The rate constant, the activation energy and the arrhenius parameter of a chemical reaction at 25°C are 3.0 × 10–4s–1,104.4 kJ mol–1 and 6.0 × 1014 s–1 respectively. The value of the rate constant as T → ∞ is
(a) 2.0 × 1018 s–1
(b) 6.0 × 1014 s–1
(c) Infinity
(d) 3.6 × 1030 s–1
Answer
B
Question. In a zero-order reaction for every 10° rise of temperature,the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become :
(a) 256 times
(b) 512 times
(c) 64 times
(d) 128 times
Answer
B
Question. The activation energy for a hypothetical reaction, A → Product, is 12.49 kcal/mole. If temperature is raised from 295 to 305, the rate of reaction increased by
(a) 60%
(b) 100%
(c) 50%
(d) 20%
Answer
B
Question. For the reaction A + B → C + D. The variation of the concentration of the products is given by the curve
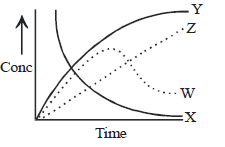
(a) Z
(b) Y
(c) W
(d) X
Answer
B
Question. Which of the following graph(s) represents exothermic reaction?
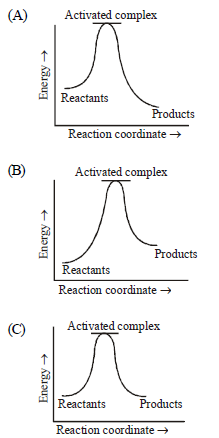
(a) (A) only
(b) (B) only
(c) (C) only
(d) (A) and (B)
Answer
A
Question. CHCl3 + Cl2 → CCl4 + HCl Rate law for above reaction will be
Rate = k[CHCl3][Cl2 ]1/2
On the basis of information provided which of the following option will be correct ?
(a) Rate law for any chemical reaction can be predicted accurately by looking at balanced chemical equation.
(b) Rate law for a chemical reaction has to determine experimentally.
(c) Either determined experimentally or obtained from balanced chemical reaction, rate law will be same.
(d) None of the above is correct.
Answer
B
Question. Consider the reaction A f B. The concentration of both the reactants and the products varies exponentially with time.Which of the following figures correctly describes the change in concentration of reactants and products with time?
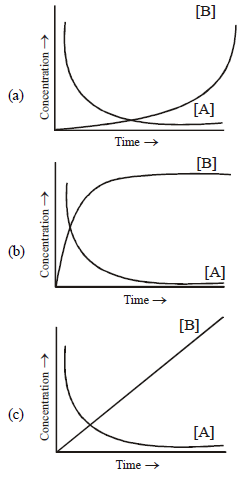
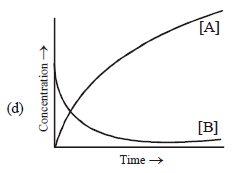
Answer
B
Question. A substance ‘A’ decomposes by a first order reaction starting initially with [A] = 2.00 M and after 200 min, [A] becomes 0.15 M. For this reaction t1/2 is
(a) 53.72 min
(b) 50.49 min
(c) 48.45 min
(d) 46.45 min
Answer
A