Please see Chapter 1 Chemical Reactions and Equations Exam Questions Class 10 Science below. These important questions with solutions have been prepared based on the latest examination guidelines and syllabus issued by CBSE, NCERT, and KVS. We have provided Class 10 Science Questions and answers for all chapters in your NCERT Book for Class 10 Science. These solved problems for Chemical Reactions and Equations in Class 10 Science will help you to score more marks in upcoming examinations.
Exam Questions Chapter 1 Chemical Reactions and Equations Class 10 Science
ONE MARK QUESTIONS
Question: N2(g) + 3H2(g) ↓ 2NH3(g), name the type of reaction.
Answer: Combination reaction.
Question: Write a balanced equation for the chemical reaction that can be characterised as precipitation reaction.
Answer: AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
It is a precipitation reaction.
Question: Which one is a chemical change: fermentation of fruit juice or diluting fruit juice?
Answer: Fermentation of fruit juice is a chemical change.
Question: Define oxidation and reduction.
Answer: Oxidation is a process in which oxygen is added or loss of electrons take place. Reduction is a process in which hydrogen is added or gain of electrons takes place.
Question: State the main difference between endothermic reaction and an exothermic reaction.
Answer: In endothermic reaction, heat is absorbed. In exothermic reaction, heat is evolved.
Question: What change in colour is observed when white silver chloride is left exposed to sunlight? State the type of chemical reaction in this change.
Answer: Grey coloured silver metal is formed and pungent smelling chlorine gas is evolved.
2AgCl(s) sunlight → 2Ag(s) + Cl2(g)
It is photochemical decomposition reaction.
Question: What happens chemically when quick lime is added to water filled in a bucket?
Answer: Calcium hydroxide (Slaked lime) is formed with evolution of heat and hissing sound.
CaO(s) + H2O(l) → Ca(OH)2(aq)
Question: Write a balanced chemical equation for the reaction between sodium chloride and silver nitrate indicating the physical state of the reactants and the products.
Answer: AgNO (aq) + NaCl (aq) →Heat AgCl (s) + NaNO (aq)
(Silver Nitrate) (Sodium Chloride) (Silver Chloride) (Sodium NItrate)
Question: Complete and balance the following equation:
Fe2O3 + Al →
Answer: Fe2O3 + 2Al → 2Fe + Al2O3
Question: Balance the following chemical equation:
Pb(NO3)2 → PbO + NO2 + O2
Answer: 2Pb(NO3)2 → Heat2PbO + 4NO2 + O2
Question: Identity the type of reaction in the following example:
Na2SO4(aq) + BaCl2(aq) → BaSO4 → + 2NaCl(ag)
Answer: Double displacement reaction.
Question: Identify the type of reaction in the following example:
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
Answer: Displacement reaction.
Question: Give an example of double displacement reaction (only with complete balanced equation).
Answer: BaCl2(aq) + H2SO4(dil.) → BaSO4(s) + 2HCl(aq)
It is a double displacement reaction.
Question: Balance the given chemical equation:
Al(s) + CuCl2(aq) → AlCl3(aq) + Cu(s)
Answer: 2Al(s) + 3CuCl2(aq)→ 2AlCl3(aq) + 3Cu(s)
Question: On what basis is a chemical reaction balanced?
Answer: Chemical equation is balanced on the basis of law of conservation of mass.
Question: Balance the given chemical equation:
FeSO4(s) Heat→ Fe2O3(s) + SO2(g) + SO3(g)
Answer: 2FeSO4(s) Heat→ Fe2O3(s) + SO2(g) + SO3(g)
Question: Identify the type of reaction in the following example:
2H2(g) + O2(g)→ 2H2O(l)
Answer: Combination reaction.
Question: On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue green. Predict the new compound formed which imparts a blue green colour to the solution.
Answer:CuO + 2HCl → CuCl2 + 2H2O
Copper chloride solution imparts blue green colour to the solution.
TWO MARKS QUESTIONS
Question: (a) Complete the following equation for the chemical reaction:
FeSO4(s)→ Heat Fe2O3 + ___ +___
(b) What happens when water is added to quicklime (CaO)? Write the chemical equation.
Answer:(a) 2FeSO4(s) → Heat Fe2O3(s) + SO2(g) + SO3(g)
(b) Slaked lime Ca(OH)2 is formed. Hissing sound and lot of heat is also produced:
CaO(s) + H2O(l) → Ca(OH)2(aq)
Question: Write the essential condition for the following reaction to take place:
2AgBr→ 2Ag + Br2
Write application of this reaction.
Answer: The reaction will take place in presence of sunlight.
This reaction is used in black and white photography.
Question: List two observations that are noticed when an iron nail is put inside copper sulphate solution. Write the chemical equation for the reaction that occurs.
Answer:The blue coloured solution will become pale green. Reddish brown metal will get deposited.
Fe(s)+ CuSO4 (aq)→ FeSO4 (aq)+ → Cu(s)
(Blue) (Pale Green) (Reddish brown)
Question: Write balanced chemical equation for the following reactions:
a. Hydrogen sulphide burns in air to give water and sulphur dioxide.
b. Barium chloride reacts in aqueous solution with zinc sulphate to give zinc chloride and barium sulphate.
Answer: a. 2H2S(g) + 3O2(g) → 2H2O(l) + 2SO2(g)
b. BaCl(aq) + ZnSO(aq) → BaSO4 (s) + ZnCl2(aq)
Question: “We need to balance a skeleton chemical equation”. Give reason to justify the statement.
Answer: We must balance a skeletal equation so as to ensure that the reaction follows ‘Law of conservation of mass’. The total mass of reactants must be equal to the total mass of products, that is why all reactions should he balanced.
Question: Name the reducing agent in the following reaction:
3MnO2 + 4A1 → 3Mn + Al2O3
State which is more reactive, Mn or Al and Why?
Answer: Al is the reducing agent. Al is more reactive than Mn.
Reason: It is because Al is displacing Mn from MnO2.
Question: Giving an example list two important information which makes a chemical equation more useful (informative).
Answer:a. It should include physical states of reactants and products.
b. It should specify conditions under which reaction takes place e.g.,
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
(Cold)
H2(g) + Cl2(g) → sunlight 2HCl(g)
Question: Consider the following chemical equation:
X + Barium chloride → Y + Sodium chloride
(White ppt)
Identify (a) X and Y (b) The type of reaction.
Answer: (a) X is silver nitrate, Y is silver chloride.
2AgNO3 (aq) BaCl2 (aq)→ 2AgCl ↓+Ba(3NO3 )2 (aq)
(White ppt)
(b) The reaction is an example of double displacement
(precipitation) reaction.
Question: (a) Write a balanced chemical equation for the process of photosynthesis.
(b) When do desert plant take up carbon dioxide and perform photosynthesis.
Answer:a. 6CO2(g) + 6H2O(l) chlorophyll→ sunlight C6H12O6(aq) + 6O2(g)
b. Desert plant take up carbon dioxide and perform photosynthesis at night.
Question: A metal is treated with dilute H2SO4. The gas evolved is collected by the method as shown in figure. Answer the following questions:
a. Name the gas liberated.
b. Name the method used for collection of gas.
c. Is the gas soluble or insoluble in water?
d. Is the gas lighter or heavier than air?
Answer: a. The gas liberated is H2.
b. It is collected by downward displacement of water.
c. The gas is insoluble in water.
d. Hydrogen gas is lighter than air.
Question: Translate the following statement into chemical equation and then balance it. “A metal in the form of ribbon burns with a dazzling white flame and changes into white powder.”
Answer: 2Mg(s) + O2(g) → Burning 2MgO(s) + Light + Heat
Question:On heating copper powder in air, the surface of copper powder becomes coated with black CuO. How can this black coating be converted into brown copper? Write chemical equation for the reaction that occurs during the colour change.
Answer:
2Cu + O2 → Heat 2CuO
(Black)
Copper oxide on heating with H2 will change back to reddish brown copper metal.
CuO(s) + H2(g) → Heat Cu(s) + H2O(g)
Question: Two reactions are given below:
a. 2KI + Cl2 → 2KCl + I2
b. 2K + Cl2 → 2KCl
Identify the type of reaction, giving justification in each case.
Answer: a. Displacement reaction because Cl2 is displacing I2 from KI solution.
b. Combination reaction because K reacts with Cl2 to form potassium chloride.
Question: What is observed when a solution of potassium iodide is added to a solution of lead nitrate? Name the type of reaction. Write a balanced chemical equation to represent the above chemical reaction.
Answer: Yellow precipitate is formed due to formation of lead iodide.
It is a precipitation as well as double displacementreaction.
Pb(NO3 ) (aq)+ 2KI (ag) → PbI2 (s) 2KNO3 (aq)
Yellow ppt
Question: Give one example of each:
a. Chemical reaction showing evolution of a gas.
b. Change in colour of a substance during chemical reaction.
Answer:
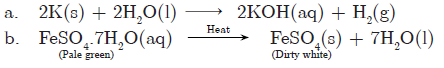
Question: Write a balanced chemical equation for the process of photosynthesis and the conditions of reaction giving physical state of all substances.
Answer:
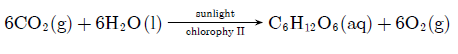
Question: It has been found that marble of Taj is getting corroded due to development of industrial areas around it. Explain this fact giving a chemical equation.
Answer: Taj is made up of CaCO3 which reacts with acid formed by pollution of SO2(g) and NO2 from Mathura refinery and other industries.
CaCO3 + 2H2SO4 → CaSO4 + CO2 + 2H2O
CaCO3 + 2HNO3 → Ca(NO3)2 + H2O + CO2
Question: Consider the chemical equations given below and answer the questions which follow:
(i) CuO + H2 → Heat Cu + H2O
(ii) ZnO + C →Heat Zn + CO
a. Name the substances that are oxidised and reduced respectively in each case,
b. Identify the reducing agent in each case.
Answer: a. H2 is getting oxidised to H2O, CuO getting reduced to Cu.
b. H2 is reducing agent in (i) reaction whereas C is reducing agent in second reaction.
Question: Write balanced chemical equations for the following reactions:
a. Silver bromide on exposure to sunlight decomposes into silver and bromine.
b. Sodium metal reacts with water to form sodium hydroxide and hydrogen gas.
Answer: a. 2AgBr(s)→ sunlight 2Ag(s)+ Br2(g)
b. 2Na(s) + 2H2O(l) → 2NaOH(l) + H2(g)
Question: What is a redox reaction? Identify the substances oxidised and the substance reduced in the following reactions:
a. MnO2+ 4HCl → MnCl2 + Cl2 + H2O
b. CuO + H2 → Cu + H2O
Answer: Redox reaction is a reaction in which oxidation and reduction takes place simultaneously.
c. HCl is the substance oxidised, MnO2 is the substance getting reduced.
d. H2 is getting oxidised, CuO is getting reduced.
Question: Identify the type of reaction from the following equations:
a. CH4 + 2O2 → CO2 + 2H2O
b. Pb(NO3)2 + 2KI → PbI2 + 2KNO3
c. CaO + H2O → Ca(OH)2
d. CuSO4 + Zn → ZnSO4 + Cu
Answer: a. Oxidation reaction
b. Double displacement reaction
c. Combination reaction
d. Displacement reaction
Question: a. What is colour of ferrous sulphate crystals? How does this colour change after heating?
b. Name the products formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this change?
Answer: a. FeSO4 ·7H2O → crystals are pale green in colour. They become dirty white on heating.
b. Ferric oxide, sulphur dioxide and sulphur trioxide are formed:
2FeSO4(s)→ Heat Fe2O3(s) + SO2(g) + SO3(g)
It is a decomposition reaction.
Question: Reaction of compound X with aluminium is used to join railway tracks or cracked machine parts.
a. Identify the compound.
b. Name the reaction.
c. Write a balanced chemical equation for the reaction.
Answer: a. The compound X is Fe2O3 (Ferric oxide) or Iron (III) oxide.
b. It is called Thermite Reaction.
c. 2Al(s) + Fe2O3(s)→ Heat Al2O3(s) + 2Fe(l)
Question: Using balanced chemical equation explain the difference between a displacement reaction and a double displacement reaction.
Answer: Displacement reaction: A reaction in which a more reactive element displaces a less reactive element from its salt solution e.g.,
2KBr(aq) + Cl2(g) → 2KCl(aq) + Br2(aq)
Double displacement reaction: A reaction in which
two compounds exchange their ions to form two new
compounds e.g.,
KOH + HNO3 → KNO3 + H2O
Question: Give an example each for thermal decomposition and photochemical decomposition reactions. Write balanced chemical equation also.
Answer:Thermal decomposition:
ZnCO3(s) →Heat ZnO(s) + CO2(g)
Photochemical decomposition:
2AgI(s) →sunlight 2Ag(s) + I2(g)
THREE MARKS QUESTIONS
Question: Decomposition reactions require energy either in the form of heat, light or electricity for breaking down the reactants. Write an equation each for decomposition reactions where energy is supplied in the form of heat, light and electricity.
Answer:
a. CaCO3(s) → Heat CaO(s) + CO2(g)
b. 2AgBr(s) → sunlight 2Ag(s) + Br2(g)
c. 2H2O(l) → Electricity 2H2(g) + O2(g)
Question: In the electrolysis of water:
a. Name the gas collected at the cathode and anode respectively.
b. Why is volume of gas collected at one electrode double than that at the other? Name this gas.
c. How will you test this gas?
Answer: a. Hydrogen is collected at the cathode, oxygen is collected at the anode.
b. It is because H2O contains hydrogen and oxygen in the ratio 2 : 1.
c. Bring a burning matchstick near the gas, if the gas burns with ‘pop’ sound, the gas is H2.
Question: State one example each characterised by following along with suitable chemical equation.
a. Change in state,
b. Evolution of gas,
c. Change in temperature.
Answer:
a. Change in state:
AgNO3(aq) + HCl(aq)→ AgCl(s) + HNO3(aq)
b. Evolution of gas:
Download more materials in free at : www.cyberworldforu.blogspot.com
CaCO3 (s) +2HCl dil →CaCl2 aq H2O(1)+ CO2 (g)
c. Change in temperature:
d. CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) + Heat
Question: Name the type of reactions represented by the following equations:
a. CaO + H2O → Ca(OH)2
b. 3BaCl2 + Al2(SO4) → 3BaSO4 + 2AlCl3
c. 2FeSO4→ Heat Fe2O3 + SO2 + SO3
Answer:
a. Combination reaction,
b. Double displacement reaction,
c. Decomposition reaction.
Question: In a schematic diagram for the preparation of hydrogen
gas as shown in the figure. What would happen if the following changes are made
a. In place of zinc granules, same amount of zinc dust is taken in the test tube?
b. Instead of dilute sulphuric, dilute hydrochloric acid is taken?
c. Sodium hydroxide is taken in place of dilute sulphuric acid and the flask is heated?
Answer:
a. Zinc dust will react faster, H2(g) will be liberated at a faster rate.
b. Same volume of H2(g) will be formed.
c. On heating Zn with NaOH, hydrogen gas will be formed at a faster rate.
Question: (a) Why is it necessary to balance a chemical equation?
(b) Write the balanced chemical equation for the following reactions:
(i) Natural gas burns in air to form carbon dioxide and water.
(ii) During respiration, glucose combines with oxygen and forms carbon dioxide and water along with the release of energy.
Answer:
a. Chemical equation must be balanced so as to follow the law of conservation of mass.
b. (i) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
(ii) C6H12O6(s) 6O2(g) 6CO2(g) 6H2O(l) Heat
Question: A green coloured hydrated metallic salt on heating loses water of crystallisation molecules and gives a gas with suffocating smell. Identify the salt and write the chemical equation for the reaction.
Answer:

Question: a. Can combination reaction be an oxidation reaction?
b. How will you test whether the gas evolved in a reaction is hydrogen?
c. Why does copper not evolve hydrogen on reacting with dilute sulphuric acid?
Answer:
a. Yes, combination reaction can be called a oxidation reaction.
b. Bring a burning splinter near the gas, if it burns with pop sound, it is hydrogen gas.
c. It is because copper is less reactive than hydrogen.
Question: Write balanced chemical equations for the following reactions:
a. Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
b. Barium chloride reacts with zinc sulphate to give zinc chloride and barium sulphate.
c. Natural gas bums in air to form carbon dioxide and water.
Answer:
a. 2H2S(g) + 3O2(g) → 2H2O(l) + 2SO2(g)
b. BaCl2(aq) ZnSO4(aq) → BaSO4(s) ZnCl2(aq)
c. CH4(g) + 2O2 → CO2(g) + 2H2O(l)
FIVE MARKS QUESTIONS
Question: Identify the type of chemical reaction in the following statement and define each of them:
a. Digestion of food in our body.
b. Rusting of iron.
c. Heating of manganese dioxide with aluminium powder.
d. Blue colour of copper sulphate solution disappears when iron filings are added to it.
e. Dilute hydrochloric acid is added to sodium hydroxide solution to form sodium chloride and water.
Answer: a. Decomposition reaction: It is a process in which a compound is broken down into simple substances.
b. Oxidation: The process in which oxygen is added or electrons are lost.
c. Displacement reaction: The reaction in which a more reactive element can displace a less reactive element from its salt solution. Oxidation and Reduction are taking place simultaneously in rusting of iron. Download more materials in free at :
d. Displacement reaction: The reaction in which a more reactive element can displace a less reactive element.
e. Neutralisation reaction: The reaction in which acid reacts with base to form salt and water.
Question: Write balanced chemical equation for the following statements:
a. NaOH solution is heated with zinc granules.
b. Excess of carbon dioxide is passed through lime water.
c. Dilute sulphuric acid is added to sodium carbonate.
d. Egg shell is dropped in hydrochloric acid,
e. Copper (II) oxide reacts with dilute hydrochloric acid.
Answer:
a. Zn(s) + 2NaOH → Na2ZnO2 + H2
b. Ca(OH)2 + 2CO2 → Ca(HCO3)2
c. Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2
d. CaCO3(s) + 2HCl(dil) → CaCl2 + H2O + CO2
e. CuO(s) + 2HC1 → CuCl2 + H2O

