Please refer to Class 10 Science Sample Paper Term 2 Set B with solutions below. The following CBSE Sample Paper for Class 10 Science has been prepared as per the latest pattern and examination guidelines issued by CBSE. By practicing the Science Sample Paper for Class 10 students will be able to improve their understanding of the subject and get more marks.
CBSE Class 10 Science Sample Paper for Term 2
SECTION – A
1. Write the next homologue of each of the following :
(a) C2H4
(b) C4H6
Ans. (a) C2H4 belongs to alkene series having general formula, CnH2n.
Thus, next homologue will be C3H2 × 3 = C3H6
(b) C4H6 belongs to alkyne series having general formula, CnH2n–2.
Thus, next homologue will be C5H2 × 5 – 2 = C5H8
2. Which element exhibits the property of catenation to maximum extent and why?
Ans. Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large number of molecules. This property is called catenation. Carbon shows catenation to maximum extent due to its small size and stronger carbon-carbon bond strength.
OR
Write the name and molecular formula of the first member of the homologous series of alkynes.
Ans. General formula for alkyne is CnH2n–2.
First member of homologous series of alkyne has the formula, C2H2 × 2–2 = C2H2 i.e., ethyne.
3. What are sperms? Name the part of male reproductive system where sperms are produced.
Ans. Sperms are male gametes. These are microscopic, motile cells. It consists of head (contains small acrosome and large nucleus), neck (contains proximal centriole towards the nucleus), middle piece (contains mitochondrial spiral) and tail (divided into main piece and end piece). Tail helps them to swim. The formation of male germ-cells or sperms takes place in the testes.
4. How is the sex of the child fixed during the fertilisation step in human beings? Explain.
Ans. Human beings have 23 pairs of chromosomes (22 pairs of autosomes +1 pair of sex chromosome). A male has one X chromosome and one Y chromosome whereas a female has two X chromosomes. Sex of a child depends on the two conditions which takes place during fertilisation :
(i) If a sperm carrying X chromosome fertilises an ovum which carries X chromosome, then the child born will be girl.
(ii) If a sperm carrying Y chromosome fertilises an ovum which carries X chromosome, then the child born will be a boy.
5. (a) Name two ozone depleting substances.
(b) What do you mean by biological magnification?
Ans. (a) Chlorofluorocarbons(CFCs) present in refrigerators, aerosol spray, propellants etc, and methane are ozone depleting substances.
(b) Biomagnification is the process of increase in amount of some toxic, non-biodegradable substances such as DDT and heavy metals in successive trophic levels of a food chain. It results in accumulation of these toxic substances in highest concentration at topmost trophic level.
OR
Define a food web. Write its significance for ecosystem.
Ans. Food web is a network of food chains which become inter-connected at various trophic levels so as to form a number of feeding connections amongst different organisms of a biotic community.
A food web maintains ecological balance by maintaining the interdependence of different organisms.
6. F, Cl and Br are the elements each having seven valence electrons. Which of these
(a) has the largest atomic radius
(b) is most reactive?
Justify your answer stating reason for each.
Ans. (a) F, Cl and Br all have seven valence electrons so, they belong to the same group. On moving down the group, the atomic size of the elements increases due to addition of extra shell at each successive element. Due to this, the average distance between the nucleus and outermost electrons increases. Thus, Br is largestin size among F, Cl and Br.
(b) Fluorine is the most reactive element because the chemical reactivity of non-metals decreases on going down the group as the size of the atom increases.Hence, the tendency to attract the incoming electrons decreases. Therefore, the tendency of atoms to gain electrons decreases due to which their reactivity decreases.
OR
How do atomic radius, valency and metallic character vary on moving?
(a) across the period
(b) down the group?
Ans. (a) On moving from left to right in a period, atomic size decreases, valency first increases from 1 to 4 then decreases to O and metallic character of elements decreases.
(b) On moving down the group, atomic size increases, valency remains the same and metallic character of elements increases.
7. (a) Why is tungsten used for making bulb filaments of incandescent lamps?
(b) Name any two electric devices based on heating effect of electric current.
Ans. (a) Tungsten is a strong metal and has high melting point (3380°C). It emits light at high temperatures (about 2500°C).
(b) Electric laundry iron and electric heater are based on heating effect of electric current.
SECTION – B
8. How does the uterus prepare itself every month to receive and nurture the growing embryo? Where does fertilization take place in human females?
Ans. Methane are ozone depleting substances. The uterus prepares itself every month to receive and nurture the growing embryo. The lining thickens and is richly supplied with blood to nourish the growing embryo. Fertilisation takes place in the oviduct or fallopian tube in females.
OR
Differentiate between self-pollination and cross-pollination.
Ans. Following are the differences between self-pollination and cross-pollination.
| Character | Self-pollination | Cross-pollination | |
| 1. | Occurrence | Occurs within a flower or between two flowers of the same plant. | Occurs between two flowers of two different plants of the same species. |
| 2. | Agent of pollination | No external agent of pollination required (usually). | External agents such as wind, water, insects and birds required. |
| 3. | Production of pollen grains | Produced in small numbers, thus no wastage of pollen grains occurs. | Produced in large numbers (usually), thus, wastage of pollen grains occurs. |
| 4. | Appearance of flowers | Flowers are not attractive (usually). | Flowers are attractive with coloured petals in case of entomophily. |
| 5. | Fragrance and nectar | Flowers do not (usually) produce scent or nectar. | Flowers generally produces scent and nectar. |
| 6. | Nature of offsprings produced | Offsprings produced have genetic make up identical to the parent plant, purity of race maintained, no variation occurs. | Offsprings produced may differ in genetic make-up, and variations occur. |
9. Describe the role of any three parts of human male reproductive system.
(a) Testes (b) Scrotum (c) Vas deferens (d) Urethra (e) Penis
Ans. Human male reproductive system consists of testes, scrotum, vas deferens, urethra and penis.
(a) Testes : The human male possesses two testes, which are the primary reproductive organs, lying outside the abdominal cavity. The two testes are themale gonads, which are the sites where male gametes, i.e., sperms are produced. The testes also produce the male sex hormone-testosterone. The testes of man produce sperms from puberty onwards, throughout his life.
(b) Scrotum: It is a pouch of skin that hangs between the legs. It is divided internally into right and left scrotal sacs by a muscular partition. The two testes lie in respective scrotal sacs. The scrotum acts as a thermoregulator and provides an optimal temperature for the formation of sperms. The sperms develop at a temperature 2 – 2.5°C lower than the normal body temperature.
(c) Vas deferens : This is a straight tube, about 40 cm long, which carries the sperms to the seminal vesicles. The sperms are stored temporarily in the seminal vesicle, where mucus and a watery alkaline fluid containing the sugar-fructose, mix with the sperms.
10. When a current carrying wire is placed in a magnetic field,
(a) what are the factors on which the magnitude of force experienced by wire depends?
(b) when is the magnitude of this force maximum?
Ans. (a) When a current carrying wire is placed in a magnetic field, it experiences a magnetic force that depends on
(i) current flowing in the conductor
(ii) strength of magnetic field
(iii) length of the conductor
(iv) angle between the element of length and the magnetic field.
(b) Force experienced by a current carrying conductor placed in a magnetic field is largest when the direction of current is perpendicular to the direction of magnetic field.
11. A coil of insulated wire is connected to a galvanometer. What would be observed if a bar magnet with its north pole towards one face of the coil is
(a) moved quickly towards it, and
(b) moved quickly away from the coil ?
Ans. If a coil of insulated wire is connected to a galvanometer and a bar magnet with north pole is towards one face of the coil when magnet is
(a) Moved quickly towards the coil : A current is induced in anti-clockwise direction in the coil with respect to the side facing the north pole of the magnet and needle of galvanometer will deflect in one direction from zero position.
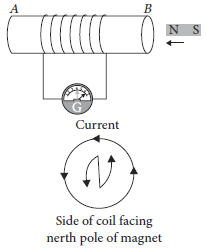
(b) Moved quickly away from coil : A current is induced in clockwise direction in the coil with respect to the side facing the north pole of the magnet and the needle of the galvanometer will deflect in opposite direction from case (a).
OR
Figure shows a small plotting compass placed above a copper wire. When there is no current in the wire, the plotting compass points towards the North.

(a) A large current is switched on in the wire. The direction of the current is shown in Figure.
(i) State what happens to the compass needle.
(ii) State what happens if the compass is placed under the wire.
(b) State and explain what is observed if there is 50 Hz alternating current in the wire.
Ans. (a) (i) The compass needle will deflect to the left and point West.
(ii) The compass needle will deflect to the right and point East.
(b) The alternating current will change the direction of the current 50 times per second. Hence, the compass needle will deflect to the left and then to the right and then back to the left 50 times per second.
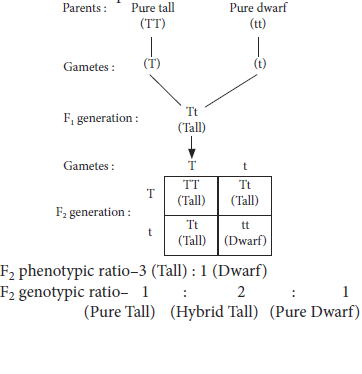
12. If we cross pure-breed tall (dominant) pea plant with pure-breed dwarf (recessive) pea plant we will get pea plants of F1 generation. If we now self-cross the pea plant of F1 generation, then we obtain pea plants of F2 generation.
(a) What will be the phenotype of F1 generation of the given pea plants?
(b) State the ratio of tall plants and dwarf plants in F2 generation.
(c) State the type of plants not found in F1 generation but appeared in F2 generation, mentioning the reason for the same.
Ans. (a) The pea plants of F1 generation will be all tall plants(Tt).
(b) The ratio of tall plants to dwarf plants in F2 generation is 3 : 1.
(c) Dwarf plants are not found in F1 generation but appeared in F2 generation. This is so because in F1 generation only dominant trait (tall) expresses itself and recessive trait (dwarf) gets suppressed. The dwarf plants appeared in F2 generation, because the traits whether dominant or recessive are independently inherited. In others words, a single copy of (T) is enough to make the plant tall, while both copies of (t) is needed for the plant to be dwarf.
13. What are trophic levels? Give an example of a food chain and state the different trophic levels in it
Ans. Trophic level is a step of food chain which are characterised by the methods of obtaining the food. The number of trophic levels is equal to the number of steps in the food chain. The common example of food chain in a terrestrial ecosystem is:
Plants → Deer → Tiger.
Plants belong to the first trophic level of the food chain. They are the producers. Deer being a herbivorous animal, feeds upon plants and constitutes the second trophic level in the food chain as the primary consumer and tiger is the secondary consumer occupying the third trophic level as it feeds upon deer.
SECTION – C
This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (a, b and c). Parts a and b are compulsory. However, an internal choice has been provided in part c.
14. Two or more resistances are connected in series or in parallel or both, depending upon whether we want to increase or decrease the circuit resistance
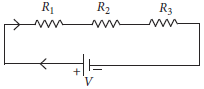
The two or more resistances are said to be connected in series if the current flowing through each resistor is same. The equivalent resistance in the series combination is given by
Rs = R1 + R2 + R3
(a) When three resistors are connected in series with a battery of voltage V and voltage drop across resistors is V1 , V2 and V3. What will be the relation between voltage and voltage drop across resistors?
(b) When the three resistors each of resistance R ohm connected in series, what is the equivalent resistance?
(c) There is a wire of length 20 cm and having resistance 20 W cut into 4 equal pieces and then joined in parallel. What is the equivalent resistance?
Ans. (a) In series combination, the total voltage is equal to the sum of voltage drop across each resistance.
Therefore V = V1 + V2 + V3
(b) Rs = R1 + R2 + R3
So, Rs = R + R + R = 3R
(c) Resistance of each wire = 20/4 = 5 Ω
Equivalent resistance in parallel
1 / Rp = 1/5 + 1/5 + 1/5 + 1/5
1 / Rp = 4/5
∴ Rp = 5/4 Ω
OR
In the given circuit calculate the total resistance of the circuit and potential drop across 12 Ω resistance.
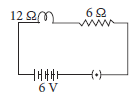
Ans. Total resistance Rs = 12 + 6 = 18 Ω
Current I = V / Rs = 6 / 18 = 0.33 A
Now potential drop (V) across 12 Ω = I × 12 Ω
= 33 × 8 = 3.96 V
15. Maximum number of electrons that can be accommodated in a shell is given by the formula : 2n2, where n is the number of the outermost shell from the nucleus.
For example,
K shell – 2 × (1)2 ⇒ 2, hence, K-shell can accommodate maximum 2 electrons.
L shell – 2 × (2)2 ⇒ 8, hence, L-shell can accommodate maximum 8 electrons.
In the modern periodic table, elements are placed according to their electronic configurations. The elements present in any group have the same number of valence electrons. The elements present in any period contain the same number of shells. The first period of the modern periodic table corresponds to the filling of electrons in the first energy shell, i.e., K-shell, first period has two elements. The second period of the periodic table corresponds to the filling of electrons in the second energy shell, i.e., L-shell, second period contains eight elements. The third, fourth, fifth, sixth and seventh periods have 8, 18, 18, 32 and 32 elements respectively.
(a) Electronic configuration of an element ‘X’ is 2, 1. What is the total number of elements present in the period to which ‘X’ belongs?
(b) Out of helium, neon, calcium and fluorine, which element has two shells and both are completely filled?
(c) The elements A, B, C and D have atomic numbers 4, 12, 17 and 19 respectively. Which pair of elements belong to the same period?
Ans. (a) ‘X’ is Li. It belongs to second period. Number of elements present in a period is 2 × n2, where n is the number of outermost shell from the nucleus.
Thus, second period has 2 × 22 i.e., 8 elements.
(b) 10Ne : K L
2 8
Both K and L shells are completely filled.
(c) Electronic configurations of
K L M N
A : 2, 2
⇒ 2nd period
B : 2, 8, 2
⇒ 3rd period
C : 2, 8, 7
⇒ 3rd period
D : 2, 8, 8, 1
⇒ 4th period
Thus, B and C belong to the same period.
OR
The elements A, B, C and D have atomic number 3, 11, 16 and 19 respectively. Which of the elements have the same number of electrons in outermost shell?
Ans. Electronic configuration of
K L M N
A : 2 1
B : 2 8 1
C : 2 8 6
D : 2 8 8 1
Thus, A, B and D have same number of electrons in outermost shell which is 1.
