Please refer to Class 12 Chemistry Sample Paper Set B with solutions below. The following CBSE Sample Paper for Class 12 Chemistry has been prepared as per the latest pattern and examination guidelines issued by CBSE. By practicing the Chemistry Sample Paper for Class 12 students will be able to improve their understanding of the subject and get more marks.
1. Experimentally it was found that a metal oxide has formula M0.98O. Metal M, is present as M2+ and M3+ in its oxide. Fraction of the metal which exists as M3+ would be
(a) 5.08%
(b) 7.01%
(c) 4.08%
(d) 6.05%
Answer
C
2. In the synthesis of glycerol from propene, the steps involved are
(a) glycerol-b-chlorohydrin and allyl chloride
(b) glyceryl trichloride and glycerol-a-chlorohydrin
(c) allyl alcohol and glycerol-b-chlorohydrin
(d) allyl alcohol and monosodium glycerolate.
Answer
C
3. A solution containing one mole per litre of each Cu(NO3)2; AgNO3; Hg2(NO3)2; is being electrolysed by using inert electrodes. The values of standard electrode potentials in volts (reduction potentials) are
Ag+/Ag = +0.80, Hg2 2+/2Hg = +0.79
Cu2+/Cu = +0.34, Mg2+/Mg = –2.37
With increasing voltage, the sequence of deposition of metals on the cathode will be
(a) Ag, Hg, Cu, Mg
(b) Mg, Cu, Hg, Ag
(c) Ag, Hg, Cu
(d) Cu, Hg, Ag
Answer
C
4. A greenish yellow gas reacts with an alkali metal hydroxide to form a halate which can be used in fire works and safety matches. The gas and halate respectively are
(a) Br2, KBrO3
(b) Cl2, KClO3
(c) I2, NaIO3
(d) Cl2, NaClO3
Answer
B
5. The formula of the complex diamminechloroido (ethylenediamine)nitro-platinum (IV) chloride is
(a) [Pt(NH3)2Cl(en)NO2]Cl2
(b) Pt[Pt(NH3)2(en)Cl2NO2]
(c) Pt[(NH3)2(en)NO2]Cl2
(d) Pt[(NH3)2(en)NO2Cl2]
Answer
A
6. For the reaction, SO2(g) + 1/2 O2(g) ⇌ SO3(g) , if Kp = Kc(RT)x where the symbols have usual meaning then the value of x is (assuming ideality)
(a) 1
(b) – 1
(c) − 1/2
(d) 1/2
Answer
C
7. In which reaction, aromatic aldehyde is treated with acid anhydride in the presence of corresponding salt of the acid to give unsaturated aromatic acid?
(a) Friedel–Crafts reaction
(b) Perkin’s reaction
(c) Wurtz reaction
(d) None of these.
Answer
B
8. Which of the following is not a function of proteins?
(a) Nail formation
(b) Skin formation
(c) Muscle formation
(d) Providing energy for metabolism
Answer
D
9. The yield of NH3 in the reaction, N2 + 3H2 ⇌ 2NH3; ΔH = –22.08 kcal is affected by
(a) change in pressure and temperature
(b) change in temperature and concentration of N2
(c) change in pressure and concentration of N2
(d) change in pressure, temperature and concentration of N2.
Answer
D
10. Lipids are
(a) nucleic acids occurring in plants
(b) proteins occurring in animals
(c) carbohydrates occurring in plants
(d) fats of natural origin.
Answer
D
11. The rate law for a reaction between the substances A and B is given by rate = k [A]n [B]m. On doubling the concentration of A and halving the concentration of B, the ratio of the new rate to the earlier rate of the reaction will be as
(a) 1/2m+n
(b) (m + n)
(c) (n – m)
(d) 2(n – m)
Answer
D
12. In SN1 (substitution nucleophilic unimolecular) reaction, racemization takes place, it is due to
(a) inversion of configuration
(b) retention of configuration
(c) conversion of configuration
(d) both (a) and (b).
Answer
D
13. Though the five d-orbitals are degenerate, the first four d-orbitals are similar to each other in shape whereas the fifth d-orbital is different from others. The fifth orbital is
(a) dx2 – y2
(b) dz2
(c) dxz
(d) dxy
Answer
B
14. Consider the following diazonium ions :

The order of reactivity towards diazo-coupling
with phenol in the presence of dil. NaOH is
(a) I < IV < II < III
(b) I < III < IV < II
(c) III < I < II < IV
(d) III < I < IV < II
Answer
B
15. Which of the following ions are colourless?
Ti3+, Sc3+, Ag+, Cd2+, Cu2+
I II III IV V
(a) I and V only
(b) II, III and IV only
(c) I, III and V only
(d) III and IV only
Answer
B
16. Which of the following compounds does not react with ethanolic KCN?
(a) Ethyl chloride
(b) Acetyl chloride
(c) Chlorobenzene
(d) Benzaldehyde
Answer
C
17. Which of the following processes is a nonspontaneous process?
(a) Dissolution of salt or sugar in water.
(b) Mixing of different gases through diffusion.
(c) Precipitation of copper when zinc rod is dipped in aqueous solution of copper sulphate.
(d) Flow of heat from a cold body to a hot body in contact.
Answer
D
18. Which of the following compounds is the most basic in aqueous medium?
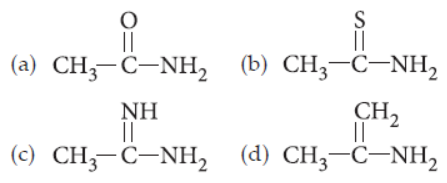
Answer
C
19. The relationship between standard reduction potential of a cell and equilibrium constant is shown by
(a) E°cell = n/0.059 logKc
(b) E°cell = 0.059/n log Kc
(c) E°cell = 0.059 n log Kc
(d) E°cell = log Kc/n
Answer
B
20. In the Liebermann’s nitroso reaction, sequential changes in the colour of phenol occurs as
(a) Brown or red → green → deep blue
(b) Red → deep blue → green
(c) Red → green → white
(d) White → red → green.
Answer
A
21. When benzenediazonium chloride in hydrochloric acid reacts with cuprous chloride, then chlorobenzene is formed. The reaction is called
(a) Gattermann reaction
(b) Perkin reaction
(c) Etard reaction
(d) Sandmeyer reaction.
Answer
D
22. Which of the following elements does not show allotropy?
(a) Nitrogen
(b) Bismuth
(c) Antimony
(d) Arsenic
Answer
B
23. The electronic configuration of Cu (II) is 3d9 whereas that of Cu (I) is 3d10. Which of the following is correct?
(a) Cu (II) is more stable.
(b) Cu (II) is less stable.
(c) Cu (I) and Cu (II) are equally stable.
(d) Stability of Cu (I) and Cu (II) depends on nature of copper salts.
Answer
A
24. Primary nitro compounds react with nitrous acid to form nitrolic acids which dissolve in NaOH giving
(a) yellow solution
(b) blue solution
(c) colourless solution
(d) red solution.
Answer
D
25. In an aqueous solution of 1 L, when the reaction
2Ag+(aq) + Cu(s) ⇌ Cu2+(aq) + 2Ag(s) reaches equilibrium, [Cu2+] = x M and [Ag+] = y M.
If volume of solution is doubled by adding water, then at equilibrium
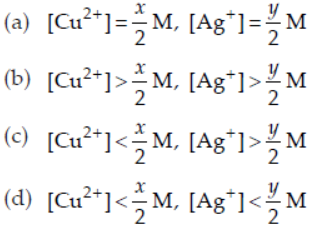
Answer
C
26. In which of the following coordination entities the magnitude of Δo (CFSE in octahedral field) will be maximum?
(a) [Co(C2O4)3]3–
(b) [Co(H2O)6]3+
(c) [Co(NH3)6]3+
(d) [Co(CN)6]3–
Answer
D
27. Which one/ones of the following reactions will yield propan-2-ol?
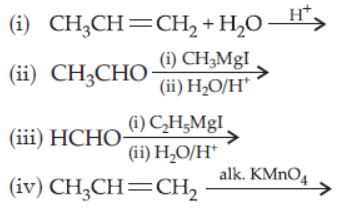
Choose the right answer.
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (ii) and (iv)
Answer
A
28. If ΔG = ΔH – TΔS and ΔG = ΔH + T[d(ΔG)dT]P then variation of EMF of a cell, with temperature T, is given by
(a) ΔS/nF
(b) – ΔS/nF
(c) ΔH/nF
(d) ΔG/nF
Answer
A
29. The radial wave equation for hydrogen atom is
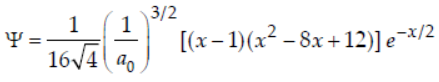
where, x = 2r / a0; a0 = radius of first Bohr orbit.
The minimum and maximum position of radial nodes from nucleus are
(a) a0, 3a0
(b) a0/2, 3a0
(c) a0/2 , a0
(d) a0/2 , 4a0
Answer
B
30. D(+)-glucose reacts with hydroxyl amine and yields an oxime. The structure of the oxime would be
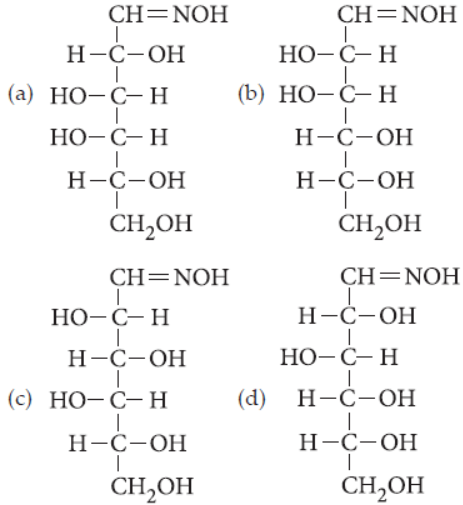
Answer
D
31. The solubility of AgCl at 20°C is 1.435 × 10–3 g/L.
The solubility product is
(a) 1.0 × 10–10
(b) 2 × 10–10
(c) 1.035 × 10–5
(d) 108 × 10–3
Answer
A
32. On heating with conc. HNO3, proteins give yellow colour. This test is called
(a) oxidising test
(b) xanthoproteic test
(c) Molisch’s test
(d) acid-base test.
Answer
B
33. The correct statement about the compounds A, B and C is
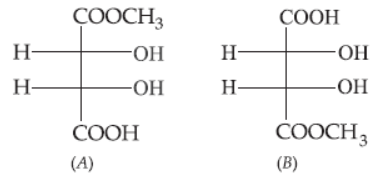
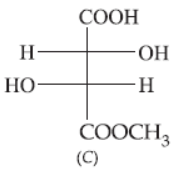
(a) A and B are identical.
(b) A and B are diastereomers.
(c) A and C are enantiomers.
(d) A and B are enantiomers.
Answer
D
34. The wave function (Ψ) of 2s is given by
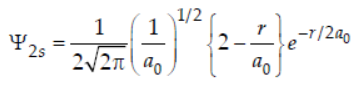
At r = r0, radial node is formed. Thus for 2s, r0 in terms of a0 is
(a) r0 = a0
(b) r0 = 2a0
(c) r0 = a0 / 2
(d) r0 = 4a0
Answer
B
35. Which of the following arrangements does not represent the correct order of the property stated against it?
(a) Sc < Ti < Cr < Mn : number of oxidation states
(b) V2+ < Cr2+ < Mn2+ < Fe2+ : paramagnetic behaviour
(c) Ni2+ < Co2+ < Fe2+ < Mn2+ : ionic size
(d) Co3+ < Fe3+ < Cr3+ < Sc3+ : stability in aqueous solution.
Answer
B
36. The pair of compounds in which both the compounds give positive test with Tollens’ reagent is
(a) glucose and sucrose
(b) fructose and sucrose
(c) acetophenone and hexanal
(d) glucose and fructose.
Answer
D
37. Which one of the following nitrophenols is the strongest acid?

Answer
A
38. When vapours, at atmospheric pressure were gradually heated from 25°C their colour was found to deepen at first and then fade as the temperature was raised above 160°C. At 600°C, the vapours were almost colourless, but their colour deepened when the pressure was raised at this temperature. The vapours were of
(a) bromine
(b) a mixture of nitrogen dioxide and dinitrogen tetraoxide
(c) pure nitrogen dioxide
(d) pure dinitrogen tetraoxide.
Answer
D
39. Acetal formation is a reversible reaction
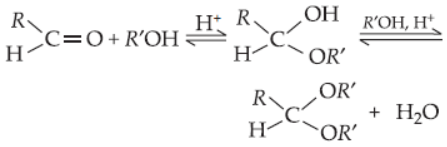
Under what conditions, the reaction can be forced to proceed only in right (forward) direction?
(a) Using excess of alcohol.
(b) Using high temperature.
(c) Using dilute acid and excess of alcohol.
(d) Using dry acid and excess of alcohol.
Answer
D
40. How many coulombs are required for the oxidation of one mole of H2O2 to O2?
(a) 93000 C
(b) 1.93 × 105 C
(c) 1.93 × 10–5 C
(d) 4.53 × 10–4 C
Answer
B
