Please refer to Acids Bases Salts MCQ Questions Class 10 Science below. These MCQ questions for Class 10 Science with answers have been designed as per the latest NCERT, CBSE books, and syllabus issued for the current academic year. These objective questions for Acids Bases Salts will help you to prepare for the exams and get more marks.
Acids Bases Salts MCQ Questions Class 10 Science
Please see solved MCQ Questions for Acids Bases Salts in Class 10 Science. All questions and answers have been prepared by expert faculty of standard 10 based on the latest examination guidelines.
Question. Calcium carbonate is the chemical formula of
(a) limestone
(b) chalk
(c) marble
(d) all (a), (b) and (c)
Answer
D
Question. On adding dilute HCl to copper oxide in a beaker, the solution turns blue-green due to formation of
(a) copper(II) hydroxide
(b) copper nitrate
(c) copper (II) chloride
(d) copper sulphate
Answer
C
Question. The chemical formula for plaster of Paris is:
(a) CaSO4.2H2O
(b) CaSO4.H2O
(c) CaSO . 1/2 H2O
(d) 2CaSO4.H2O
Answer
C
Question. A visually challenged student, has to perform a lab test to detect the presence of acid in a given solution. The acid-base indicator preferred by him will be:
(a) Blue litmus
(b) Clove oil
(c) Red cabbage extract
(d) Hibiscus extract
Answer
B
Question. If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
(a) Wash the hand with saline solution.
(b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogencarbonate.
(c) After washing with plenty of water, apply solution of sodium hydroxide on the hand.
(d) Neutralise the acid with a strong alkali.
Answer
B
Question. Sodium hydrogencarbonate when added to acetic acid evolves a gas. Which of the following statements is true about the gas evolved?
(i) It turns lime water milky.
(ii) It extinguishes a burning splinter.
(iii) It dissolves in a solution of sodium hydroxide.
(iv) It has a pungent odour.
(a) (i) and (ii)
(b) (i), (ii) and (iii)
(c) (ii), (iii) and (iv)
(d) (i) and (iv)
Answer
B
Question. Human body works within the pH range of
(a) 7.0 to 7.8
(b) 4.5 to 5.6
(c) 13.0 to 14.0
(d) 1.2 to 2.2
Answer
A
Question. A basic solution could have a pH of
(a) 1
(b) 11
(c) 7
(d) 2
Answer
D
Question. Which of the following gives the correct increasing order of acidic strength?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Answer
A
Question. Fruit juices, such as orange juice, contain
(a) boric acid
(b) citric acid
(c) sulphuric acid
(d) nitric acid
Answer
B
Question. Common salt, besides being used in kitchen, can also be used as the raw material for making
(i) washing soda (ii) bleaching powder
(iii) baking soda (iv) slaked lime
(a) (i) and (ii)
(b) (i), (ii) and (iv)
(c) (i) and (iii)
(d) (i), (iii) and (iv)
Answer
C
Question. Which of the following salts does not contain water of crystallisation?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer
B
Question. A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer
D
Question. In an attempt to demonstrate electrical conductivity through an electrolyte, the alongside apparatus was set up. Which among the following statement(s) is(are) correct? (Diagram)
(i) Bulb will not glow because electrolyte is not acidic
(ii) Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only
Answer
C
Question. Washing soda is obtained from the recrystallization of sodium carbonate. How is sodium carbonate obtained from baking soda?
(a) By heating the baking soda
(b) By reacting the baking soda with base
(c) By reacting the baking soda with acid
(d) By adding water to baking soda
Answer
A
Question. When water of crystallization is removed from copper sulphate solution, how does the colour of the salt change?
(a) From white to blue
(b) From white to red
(c) From blue to red
(d) From blue to white
Answer
D
Question. Which of the following solutions will turn phenolphthalein pink?
(a) HCl(aq)
(b) CO2(aq)
(c) KOH(aq)
(d) H2SO4(aq)
Answer
C
Question. Identify the correct representation of reaction occurring during chlor-alkali process.
(a) 2NaCl(l) + 2H2O(l) 2NaOH(l) + Cl2(g) + H2(g)
(b) 2NaCl(aq) + 2H2O(aq) 2NaOH(aq) + Cl2(g) + H2(g)
(c) 2NaCl(aq) + 2H2O(l) 2NaOH(aq) + Cl2(aq) + H2(aq)
(d) 2NaCl (aq) + 2H2O (l) 2NaOH (aq) + Cl2(g) + H2(g)
Answer
D
Question. Which of the following statements is true for acids?
(a) Bitter and change red litmus to blue
(b) Sour and change red litmus to blue
(c) Sour and change blue litmus to red
(d) Bitter and change blue litmus to red
Answer
C
Question. The acid having highest hydrogen ion concentration is one with
(a) pH = 2.5
(b) pH = 1.8
(c) pH = 7
(d) pH = 10
Answer
B
Question. The pH of the gastric juices released during digestion is:
(a) less than 7
(b) more than 7
(c) equal to 7
(d) equal to 0
Answer
A
Question. The chemical reaction shows the reactants for the formation of baking soda.
NaCl + H2O + CO2 + NH3 → X + Y
What are X and Y?
(a) X: NH3Cl; Y: NaHCO3
(b) X: NH4Cl; Y: NaHCO3
(c) X: HCl; Y: NaHCO3
(d) X: NH4Cl; Y: NaHCO2
Answer
B
Question. Incorrect statement about acids is/are
(a) they have sour taste
(b) they may change the colour of indicator
(c) they change the colour of blue litmus to red
(d) they change the colour of red litmus to blue
Answer
D
Question. CuO + (X) → CuSO4 + H2O. Here (X) is
(a) CuSO4
(b) HCl
(c) H2SO4
(d) HNO3
Answer
C
Question. The pH of a solution is 5.0. Its hydrogen ion concentration is decreased by 100 times, the solution will be:
(a) more acidic
(b) basic
(c) neutral
(d) unaffected
Answer
C
Question. A solution turns red litmus blue, its pH is likely to be:
(a) 1
(b) 4
(c) 5
(d) 10.
Answer
D
Question. A solution ‘X’ reacts with crushed egg shells to give a gas which turns lime water milky. The solution contains:
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl.
Answer
B
Question. Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic
Answer
C
Question. Which of the following will turn red litmus blue
(a) Mg(OH)2
(b) Citric acid
(c) Carbonic acid
(d) Acetic acid
Answer
A
Question. When small amount of acid is added to water, the phenomenon which occur are
(A) Dilution
(B) Neutralisation
(C) Formation of H3O+
(D) Salt formation
The correct statements are
(a) (A) and (C)
(b) (B) and (D)
(c) (A) and (B)
(d) (C) and (D)
Answer
A
Question. You are having five solutions A, B, C, D and E with pH values as follows:
A = 1.8, B = 7, C = 8.5, D = 8 and E = 5
Which solution would be most likely to liberate hydrogen with magnesium powder?
(a) Solution A and B
(b) Solution A
(c) Solution C
(d) All of the above
Answer
B
Question. The correct statement regarding universal indicator is
(a) it is an indicator having pH = 7
(b) it gives blue colour at pH = 3
(c) it becomes colourless at pH = 7
(d) it gives orange colour at pH = 3
Answer
D
Question. Which of the following will conduct electricity?
(a) Glucose solution
(b) Ethanol solution
(c) Acetic acid solution
(d) Dry HCl(g)
Answer
C
Question. The pH of a solution is 4.0. What should be the change in the hydrogen ion concentration of the solution, if its pH is to increased to 5.0.
(a) decreases to 1/10 of its original concentration
(b) halved
(c) doubled
(d) increases by 10 times
Answer
A
Question. What is the use of washing soda?
(a) To lower the temperature of the water
(b) To change the state of water
(c) To make the water alkaline
(d) To remove the permanent hardness of water
Answer
D
Question. Which solution will change blue litmus to red?
(a) NaOH(aq)
(b) H2SO4(aq)
(c) KCl(aq)
(d) NH4OH(aq)
Answer
B
Question. Lime water reacts with chlorine to give
(a) bleaching powder
(b) baking powder
(c) baking soda
(d) washing soda
Answer
A
Question. Nettle sting is a natural source of which acid?
(a) Methanoic acid
(b) Lactic acid
(c) Citric acid
(d) Tartaric acid
Answer
A
Question. A student learns that when sodium chloride reacts with water, it forms sodium hydroxide.
Which type of reaction results in the formation of sodium hydroxide?
(a) Neutralization reaction
(b) Displacement reaction
(c) Combination reaction
(d) Decomposition reaction
Answer
D
Question. Which of the following is the best possible application of calcium oxychloride?
(a) To make the water soft
(b) To reduce the pH of water
(c) To disinfect the water
(d) To change the state of water
Answer
C
Question. Which is a soluble base in water?
(a) Cu(OH)2
(b) Fe(OH)3
(c) Zn(OH)2
(d) KOH
Answer
D
Question. In general, salts
(a) are ionic compounds.
(b) contain hydrogen ions.
(c) contain hydroxide ions.
(d) turn blue litmus red.
Answer
A
Question. The equation shows the reaction of hydrochloric acid with sodium hydroxide.
HCl+ NaOH → NaCl + H2O
If the pH of the salt is 7, what are the positive and negative radicals in the salt?
(a) Na – negative radical; Cl – negative radical
(b) Na – positive radical; Cl – negative radical
(c) Na – positive radical; Cl – positive radical
(d) Na – negative radical; Cl – positive radical
Answer
B
Question. A scientist in a chemistry lab wants to make salt of pH 5.5 using acid and base. The table shows the acid and base present in the lab.

Which of the acid and base he should use for the reaction?
(a) CH3COOH and NaOH
(b) HCl and NaOH
(c) HCl and NH4OH
(d) H2CO3 and NaOH
Answer
C
Question. Which of the following properties is closely related to acids?
(a) Contain the hydroxide ion
(b) Bitter taste
(c) Salty taste
(d) Sour taste
Answer
D
Question. A base can be prepared by the reaction between
(a) an active non-metal and water.
(b) a gas and water.
(c) a sulphide and water.
(d) an active metal and water.
Answer
D
Question. Which of the following is (are) true when HCl(g) is passed through water?
(i) It does not ionise in the solution as it is a covalent compound.
(ii) It ionises in the solution.
(iii) It gives both hydrogen and hydroxyl ion in the solution.
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule.
(a) (i) only
(b) (iii) only
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
C
Question. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) Temperature of the solution decreases
(ii) Temperature of the solution increases
(iii) Temperature of the solution remains the same
(iv) Salt formation takes place
(a) (i) and (iv)
(b) (i) and (iii)
(c) (ii) only
(d) (ii) and (iv)
Answer
D
Question. When hydrogen chloride gas is prepared on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb Cl– ions from the evolved gas
Answer
C
Question. What is formed when zinc reacts with sodium hydroxide?
(a) Zinc hydroxide and sodium
(b) Sodium zincate and hydrogen gas
(c) Sodium zinc-oxide and hydrogen gas
(d) Sodium zincate and water
Answer
B
Question. A student learns that plants grow best when the pH of the soil is slightly acidic. Which range of pH is most suited for plant growth?
(a) 11 – 14
(b) 5.5 – 7
(c) 1 – 3
(d) 7 – 9
Answer
B
Question. A sting from insect A has pH of 6. The table shows the pH of four substances.
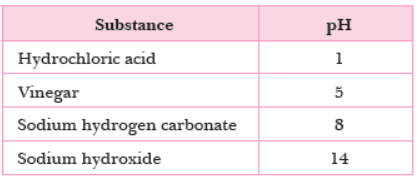
Which substance is used to treat the sting?
(a) Vinegar
(b) Sodium hydroxide
(c) Sodium hydrogen carbonate
(d) Hydrochloric acid
Answer
C
Question. Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Answer
D
Question. Brine is an
(a) aqueous solution of sodium hydroxide
(b) aqueous solution of sodium carbonate
(c) aqueous solution of sodium chloride
(d) aqueous solution of sodium bicarbonate
Answer
C
Question. Sodium carbonate is a basic salt because it is a salt of
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Answer
D
Question. Alkalis are
(a) acids, which are soluble in water
(b) acids, which are insoluble in water
(c) bases, which are insoluble in water
(d) bases, which are soluble in water
Answer
D
Question. Which of the following statements is correct about an aqueous solution of an acid and or a nbase?
(i) Higher the pH, stronger the acid (ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base (iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
D
Question. The apparatus given in the adjoining figure was set up to demonstrate electrical conductivity. (Diagram)
Which of the following statement(s) is (are) correct?
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because HCl is a strong acid and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it
depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only
Answer
C
Question. A student makes an arrangement to test the electrical conductivity of distilled water as shown.

The student observes that the bulb does not glow. What could be the reason the bulb does not glow?
(a) The water never conducts electricity.
(b) The bulb needs DC source to glow.
(c) The graphite is bad conductor of electricity.
(d) The distilled water does not have ions present in it.
Answer
D
Question. The pH values of four solutions on a pH scale are shown below.

Which solutions are alkaline in nature?
(a) A and B
(b) A and D
(c) C and D
(d) B and C
Answer
C
Question. Tooth enamel is made up of
(a) calcium phosphate
(b) calcium carbonate
(c) calcium oxide
(d) potassium
Answer
A
Question. Rain is called acid rain when its:
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
Answer
C
In the following question, a statement of Assertion
(A) is followed by a statement of Reason (R). Answer these questions by selecting appropriate option given below:
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
(b) Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of Assertion (A)
(c) Assertion (A) is true but Reason (R) is false.
(d) Assertion (A) is false but Reason (R) is true.
Question. Assertion (A): When common salt is kept open, it absorbs moisture from the air.
Reason (R): Common salt contains magnesium chloride.
Answer
A
Question. Assertion (A): Baking soda creates acidity in the stomach.
Reason (R): Baking soda is alkaline.
Answer
D
Question. Assertion (A): On adding H2SO4 to water the resulting aqueous solution get corrosive.
Reason (R): Hydronium ions are responsible for corrosive action.
Answer
A
Question. Assertion (A): H2CO3 is a strong acid.
Reason (R): A strong acid dissociates completely or almost completely in water.
Answer
D
Question. Assertion (A): Ammonia solution is an alkali.
Reason (R): Ammonia solution turns blue litmus paper red.
Answer
C
Question. Assertion (A): Sodium hydroxide reacts with zinc to produce hydrogen gas.
Reason (R): Acids reacts with active metals to produce hydrogen gas.
Answer
B
Question. Assertion (A): When zinc is added to dilute hydrochloric acid, hydrogen is given off.
Reason (R): Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms.
Answer
B
Question. Assertion (A): To dilute concentrated sulphuric acid water is added to the acid slowly.
Reason (R): A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.
Answer
A
Question. Assertion (A): HCl produces hydronium ions (H3O+) and chloride ions (Cl–) in aqueous solution.
Reason (R): In presence of water, basic give H+ ions.
Answer
C
Question. Assertion (A): In water, Hydrochloric acid behaves as a weak monobasic acid.
Reason (R): In water, Hydrochloric acid acts as a proton donor.
Answer
D
(a) Both ‘A’ and ‘R’ are true and ‘R’ is correct explanation of the assertion.
(b) Both ‘A’ and ‘R’ are true but ‘R’ is not correct explanation of the assertion.
(c) ‘A’ is true but ‘R’ is false.
(d) ‘A’ is false but ‘R’ is true.
Question. Assertion: pH of HCl solution in our stomach is about 2.
Reason: HCl in our stomach helps in digestion.
Answer
B
Question. Assertion: If the pH inside the mouth decreases below 5.5, the decay of tooth enamel begins.
Reason: The bacteria present in mouth degrades the sugar and left over food particles and produce acids that remains in the mouth after eating.
Answer
A
Question. Assertion: pH = 7 signifies pure water.
Reason: At this pH, [H+] = [OH–] = 10–7.
Answer
D
Question. Assertion: pH = 11 is strongly basic, pH=7 is neutral.
Reason: pH = 5 is weak acid and pH= 2 is strong acid.
Answer
B
Question. Assertion: Higher the pH, less will be H3O+ concentration in the solution.
Reason: pH of blood is 9.36 to 9.42.
Answer
C



