Please refer to The p – Block Elements MCQ Questions Class 12 Chemistry below. These MCQ questions for Class 12 Chemistry with answers have been designed as per the latest NCERT, CBSE books and syllabus issued for the current academic year. These objective questions for The p – Block Elements will help you to prepare for the exams and get more marks.
The p – Block Elements MCQ Questions Class 12 Chemistry
Please see solved MCQ Questions for The p – Block Elements in Class 12 Chemistry. All questions and answers have been prepared by expert faculty of standard 12 based on latest examination guidelines.
Question. Which element is used in the preparation of pesticides ?
(a) Arsenic
(b) Bismuth
(c) Antimony
(d) Nitrogez
Answer
A
Question. Which of the following phosphorus is the most reactive?
(a) Red phosphorus
(b) White phosphorus
(c) Scarlet phosphorus
(d) Violet phosphorus
Answer
B
Question. If NO2 (N2O4 ) is dissolved in NaOH, we get solution of
(a) NaNO2
(b) NaNO3
(c) mixture of NaNO2 and NaNO2
(d) NaNO4
Answer
C
Question. The nitrite ion in represented by
I. [O-N=O]Θ II. [N=O-O]Θ
Which of the structures represents possible resonance fon-ns of this ion ?
(a) Only I
(b) Only II
(c) Both I and II
(d) Neither I and I
Answer
A
Question. Which has the smallest size ?
(a) Na+
(b) Mg2+
(c) AI3+
(d) p5+
Answer
D
Question. The atomicity of phosphorus is X and the PPP bond angle in the molecule is Y. What are X and Y?
(a) X = 4, Y = 90°
(b) X = 4, Y = 60°
(c) X = 3, Y = 120°
(d) X = 2, Y = 180°
Answer
B
Question. Which of the following is not a peroxy acid?
(a) Perphosphoric acid
(b) Pernitric acid
(c) Perdisulphuric acid
(d) Perchloric acid
Answer
D
Question. The product formed in the reaction of SOCI2 with white phosphorus is
(a) PCI3
(b) SO2Cl2
(c) SCl2
(d) POCI3
Answer
A
Question. Solid N2O5 is
(a) ionic
(c) coordinate covalent
(b) covalent
(d) metallic
Answer
A
Question. The bond angle in NF3 (102.3°) is smaller than NH3 (107.2°). This is because of
(a) large size of F compared of H
(b) large size of N compared of F
(c) opposite polarity of N in the two molecules
(d) small size of H compared to N
Answer
C
Question. Which is the anhydride of nitric acid ?
(a) NO
(b) NO2
(c) N2O3
(d) N2O5
Answer
D
Question. An element belongs to group 15 and third period of the periodic table. Its electronic configw-ation will be
(a) ls22s22p3
(b) ls22s22p4
(c) ls22s22p63p23p3
(d) ls22s22p63p23p2
Answer
C
Question. Which one of the following is least covalent in nature?
(a) NF3
(b) BiF3
(c) PF3
(d) SbF3
Answer
B
Question. Ammonia is dried over
(a) slaked lime
(b) calcium chloride
(c) phosphorus pentoxide
(d) quicklime
Answer
D
Question. The statements that is not con-ect is
(a) hypophosphorous acid reduces silver nitrate to silver
(b) in solid state PCI5 exists as [PCI4]+ [PCI6]–
(c) pure phosphine is non-inflammable
(d) phosphorus acid on heating disproportionates to give metaphosphoric acid and phosphine
Answer
C
Question. Which of the following properties is not shown by NO?
(a) It is diamagnetic in gaseous state
(b) It is a neutral oxide
(c) It combines with oxygen to form nitrogen dioxide
(d) Its bond order is 2.5
Answer
A
Question. Extrapure N2 canbeobtainedbyheating
(a) NH3 with CuO
(b) NH4NO3
(c) (NH4)2Cr2O7
(d) Ba(N3)2
Answer
D
Question. Concentrated mtr1c acid, upon long standing, turns yellow-brown due to the fonnation of
(a) NO
(b) NO2
(c) N2O
(d) N2O4
Answer
B
Question. Which of the following oxides, at the same concentration when dissolved in water, results in the most acidic solutions ?
(a) CO2
(b) B2O3
(c) N2H5
(d) Li2O2
Answer
C
Question. NO2 is not obtained on heating
(a) AgNO3
(c) Cu(NO3)2
(b) KNO3
(d) Pb(NO3)2
Answer
B
Question. The reaction of white phosphorus with aqueous NaOH gives phosphine along with another phosphorus containing compound. The reaction type, the oxidation states of phosphorus in phosphine and the other product are respectively
(a) redox reaction, – 3 and – 5
(b) redox reaction, 3 and + 5
(c) disproportionation reaction, – 3 and + 5
(d) disproportionation reaction, – 3 and + 3
Answer
C
Question. The least stable hydride of 15th group elements is
(a) NH3
(b) PH3
(c) AsH3
(d) SbH3
(e) BiH3
Answer
E
Question. In which of the following, NH3 is not used?
(a) Tollen ‘s reagent
(b) Nessler’s reagent
(c) Group reagent for the analysis of IV group basic radicals
(d) Group reagent for the analysis of m group basic radicals
Answer
B
Question. The reaction of P4 with aqueous NaOH gives
(a) P(OH)3
(b) P2O5
(c) P(OH)5
(d) PH3
Answer
D
Question. Which one of the following oxides of nitrogen dimerises into a colourless solid/liquid on cooling ?
(a) N2O
(b) NO
(c) N2O3
(d) NO2
(e) N2O5
Answer
D
Question. Phosphorns pentoxide is widely used as
(a) bleaching agent
(b) dehydrating agent
(c) oxidising agent
(d) reducing agent
Answer
B
Question. Given are H3PO2 , H3PO3, H3PO4 and H4P2O7 .
Which of the above oxoacids results into two series of salts ?
(a) H3PO2
(b) H3PO3
(c) H3PO4
(d) H4P2O7
Answer
B
Question. The correct order of acidic nature of oxides is in the order
(a) NO < N2O < N2O3 < NO2 < N2O5
(b) N2O < NO < N2O3 < NO2 < N2O5
(c) N2O5 < NO2 < N2O3 < NO < N2O
(d) N2O5 < N2O3 < NO2 < NO < N2O
Answer
B
Question. Match the Column I (Molecules) with Colunm II (Boiling points) and select the correct answer.

Codes
A B C D E
(a) 3 2 5 4 1
(b) 5 3 2 4 1
(c) 1 4 5 2 3
(d) 1 2 3 4 5
Answer
B
Question. Among the following, the number of compounds that can react with PCI5 to give POCl3 is O2, CO2, SO2 , H2O ,H2SO4, P4O10 .
(a) 1
(b) 2
(c) 3
(d) 4
Answer
D
Question. The structural formula of hypophosphorous acid is
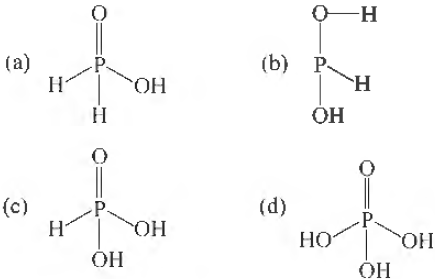
Answer
A
Question. The molecule BF3 and NF3 both are covalent compounds, but BF3 is non-polar and NF3 is polar.
The reason is that
(a) boron is a metal and nitrogen is a gas in uncombined state
(b) BF3 bonds have no dipole moment whereas NF3 bond have dipole moment
(c) atomic size of boron is smaller than that of nitrogen
(d) BF3 is symmetrical molecule whereas NF3 is unsymmetrical
Answer
D
Question. Soid PCl5 exists as
(a) PCl+4
(b) PCI5
(c) PCl+4 and PCI–6
(d) PCI–6
Answer
C
Question. The reaction ofP4 with X leads selectively to P4O6 . The Xis
(a) dry O2
(b) a mixture of O2 and N2
(c) moist O2
(d) O2 in the presence of aqueous NaOH
Answer
B
Question. The catalyst used in the manufacture of H2SO4 by contact process is
(a) V2O3
(b) V2O5
(c) FeO
(d) Cu
Answer
B
Question. SO2 doesnotactas
(a) bleaching agent
(b) oxidising agent
(c) reducing agent
(d) dehydrating agent
Answer
D
Question. The element evolving two different gases on reaction with cone. sulphuric acid is
(a) P
(b) C
(c) Hg
(d) S
(e) Sn
Answer
B
Question. The most abundant element in the earth crust is
(a) O
(b) Si
(c) H
(d) C
Answer
A
Question. Which of the following oxyacids of phosphorus is a reducing agent and monobasic ?
(a) H3PO2
(b) H3PO3
(c) H3PO4
(d) H4P2O6
Answer
A
Question. Hydrolysis of NCI3 gives NH3 and X. Which of the following is X ?
(a) HCIO4
(b) HCIO3
(c) HOCI
(d) HCIO2
Answer
C
Question. Thebasicity of H3PO4 is
(a) 2
(b) 3
(c) 4
(d) 5
Answer
B
Question. H3PO3 has ………. non-ionisable P- Hbonds.
(a) 3
(b) 1
(c) 2
(d) None of these
Answer
B
Question. Which of the following(s) when heated give nitrogen gas?
(a) (NH4)2 Cr2O7
(b) Ba(N3)2
(c) NH4NO3
(d) Both (a) and (b)
Answer
D
Question. Liquor ammonia is
(a) ammonium hydroxide
(b) liquefied ammonia gas
(c) concentrated solution of NH3 in water
(d) a solution of NH3 in alcohol
Answer
C
Question. Liquor ammonia bottles are opened only after cooling. This is because
(a) it is a mild explosive
(b) it generates high vapour pressure
(c) Both (a) and (b)
(d) it is a lachrymatory
Answer
C
Question. Industrial name of H2S2O7 is
(a) pyrosulphuric acid
(b) Marshall ‘s acid
(c) oleum
(d) All of these
Answer
C
Question. Bleaching action of SO2 is due to its
(a) oxiclising property
(b) acidic property
(c) basic property
(d) reducing property
Answer
D
Question. If an allotropic form changes slowly to a stable form. It is called
(a) enantiotropy
(b) dynamic allotropy
(c) monotropy
(d) None of these
Answer
C
Question. Which of the element of nitrogen family produce maximum number of oxyacids ?
(a) N
(b) P
(c) As
(d) Sb
Answer
B
Question. Which of the following pairs is obtained on heating anunonium dichromate ?
(a) N2 and H2O
(b) N2O and H2O
(c) NO2 and H2O
(d) NO and NO2
Answer
A
Question. As the number of – OH groups increases in hypophosphorus acid, phosphorus acid and phosphoric acid, the acidic strength
(a) increases
(b) decreases
(c) remains nearly same
(d) remains appropriately same
Answer
C
Question. Pb reacts with dilute HNO3 gives
(a) NO
(b) NH4NO3
(c) N2O5
(d) NO2
Answer
A
Question. Sodium pyrophosphate is represented by which of the following fonnula ?
(a) Na2P2O4
(b) Na4P2O5
(c) Na4P2O7
(d) Na2P2O5
Answer
C
Question. What are the products obtained when ammonia is reacted with excess chlorine ?
(a) N2 and NCI3
(c) N2 and NH4Cl
(b) N2 and HCl
(d) NCI3 and HCl
Answer
D
Question. Correct order of decreasing thermal stability is as
(a) NH3 > PH3 > AsH3 > SbH3
(b) PH3 > NH3 > AsH3 > SbH3
(c) AsH3 > PH3 > NH3 > SbH3
(d) SbH3 > AsH3 > PH3 > NH3
Answer
A
Question. Which one of the following pentafluorides cannot be formed?
(a) PF5
(b) AsF5
(c) SbF5
(d) BiF5
Answer
D
Question. Which of the following forms vortex ring?
(a) P2O5
(b) PH3
(c) NH3
(d) P4O10
Answer
B
Question. Reaction of HNO3 with I, S, P and C gives respectively
(a) HIO3 , H2SO4, H3PO4 and CO2
(b) HIO3 , H2SO4 , H3PO3 and CO2
(c) HIO2 , H2SO4, H3PO4 and CO
(d) I2O5 , SO2 , P2O and CO2
Answer
A
Question. Red P can be obtained by white P by
(a) heating it with a catalyst in an inert atmosphere
(b) distilling it in an inert atmosphere
(c) dissolving it in CS2 and crystallising
(d) melting it and pouring the liquid into water
Answer
A
Question. The treatment of Cu with dilute HNO3 gives
(a) N2O
(b) NO
(c) NH4+
(d) NO2
Answer
B
Question. NaNH2 + N2O → X + NaOH + NH3 .What is the X ?
(a) N3Ni
(c) NaN3
(b) Na3N
(d) None of these
Answer
C
Question. The sides of safety matches contains
(a) red phosphorus + sand powder
(b) P4S3
(c) Ca3 (PO)4 + glass pieces
(d) KClO3 , KNO3, sulphur + antimony
Answer
A
Question. Pnicogens are the elements of group
(a) 15
(b) 13
(c) 8
(d) zero
Answer
A
Question. In the electrothermal process, the compound displaced by silica from calcium phosphate is
(a) calcium phosphide
(b) phosphine
(c) phosphorus
(d) phosphorus pentoxide
Answer
D
Question. The catalyst used in the manufacture of anunonia is
(a) V2O5
(b) Pt
(c) Fe
(d) Ni(CO)4
Answer
C
Question. The decreasing values of bond angles from NH3 (107° )to SbH3 (91° )down the group-15 of the periodic table is due to
(a) increasing bp-bp repulsion
(b) increasing p-orbital character in sp3
(c) decreasing lp-bp repulsion
(d) decreasing electronegativity
Answer
D
Question. What is the product fonned when phosphorus trioxide is dissolved in water ?
(a) HPO3
(b) H3PO4
(c) H3PO3
(d) HPO2
Answer
C
Question. P4 + 3NaOH + 3H2O → A + 3NaH2PO2 , here A is
(a) NH3
(b) PH3
(c) H3PO4
(d) H3PO3
Answer
B
Question. The following are some statements related to VA group hydrides.
I. Reducing property increases from NH3 to BiH3.
II. Tendency to donate lone pair decreases from NH3 to BiH3.
III. Thermal stability of hydrides decreases from NH3 to BiH3.
IV. Bond angle of hydrides decreases form NH3 to BiH3.
The con-ect statements are
(a) I, II, III and IV
(b) I, III and IV
(c) I, II and IV
(d) I and IV
Answer
A
Question. Zinc and cold dil. HNO3 reacts to produce
(a) NO
(b) NO2
(c) NH4NO3
(d) ZnNO3
Answer
C
Question. Hypophosphorus acid, H3PO2 is
(a) a monobasic acid
(b) a tribasic acid
(c) a dibasic acid
(d) not acidic at all
Answer
A