Please refer to The d – and f – Block Elements MCQ Questions Class 12 Chemistry below. These MCQ questions for Class 12 Chemistry with answers have been designed as per the latest NCERT, CBSE books and syllabus issued for the current academic year. These objective questions for The d – and f – Block Elements will help you to prepare for the exams and get more marks.
The d – and f – Block Elements MCQ Questions Class 12 Chemistry
Please see solved MCQ Questions for The d – and f – Block Elements in Class 12 Chemistry. All questions and answers have been prepared by expert faculty of standard 12 based on latest examination guidelines.
Question. Zn does not show variable valency because of
(a) complete d-subshell
(b) inert pair effect
(c) 4s2-subshell
(d) None of these
Answer
A
Question. The oxidation number of Mn in the product of alkaline oxidative fusion of MnO2 is
(a) 2
(b) 3
(c) 4
(d) 6
Answer
D
Question. Among the following, the coloured compound is
(a) CuCl
(b) K3[Cu(CN)4]
(c) CuF2
(d) [Cu(CH3CN)4]BF4
Answer
C
Question. Which of the following ions is colourless in solution ?
(a) V3+
(b) Cr3+
(c) Co2+
(d) Sc3+
Answer
D
Question. Due to lanthanide contraction
(a) Fe, Co, Ni have equal size
(b) Zr and Hf have equal size
(c) all ƒ-block ions have equal size
(d) all isoelectric ions have equal size
Answer
B
Question. A compound in which a metal ion Mx+ (Z = 25)has a spin only magnetic moment of ✓24 BM. The number of unpaired electrons in the compound and the oxidation state of the metal ion are respectively
(a) 4 and 2
(b) 5 and 3
(c) 3 and 2
(d) 4 and 3
(e) 3 and 1
Answer
D
Question. Stainless steel has iron and
(a) Cr
(b) Cu
(c) Co
(d) Zn
Answer
A
Question. An ƒ-shell containing 6 unpaired electrons can exchange
(a) 6 electrons
(b) 9 electrons
(c) 12 electrons
(d) 15 electrons
Answer
D
Question. Mercury is a liquid metal because
(a) it has a completely filled s-orbital
(b) it has a small atomic size
(c) it has a completely filled d-orbital that prevents d-d overlapping of orbitals
(d) it has a completely filled d-orbital that causes d-d overlapping
Answer
C
Question. The following two reactions HNO3 with Zn are given as (equations are not balanced)
I. Zn+ cone. HNO3 → Zn (NO3)2 + X + H2O (A)
II. Zn + di!. HNO3 → Zn (NO3)2+ Y + H2O (B)
In reactions I and II, the compounds X and Y respectively, are
(a) NO2 and NO
(c) NO and NO2
(b) NO2and NO2
(d) NO2 and NH4NO3
Answer
D
Question. The lanthanoid which exhibits + 4 oxidation
(a) Pm
(b) Sm
(c) Ce
(d) Gd
Answer
C
Question. The magnetic moment of a transition metal ion is √15 BM. Therefore, the number ofunpaired electrons present in it, is
(a) 3
(b) 4
(c) 1
(d) 2
Answer
A
Question. ‘Hydride gap’ is referred to which region of the periodic table ?
(a) Groups 3, 4 and 5
(b) Groups 5, 6 and 7
(c) Groups 4, 5 and 6
(d) Groups 7, 8 and 9
(e) Groups 6, 7 and 8
Answer
D
Question. Among the following the compound that is both paramagnetic and coloured is
(a) K2Cr2O7
(b) (NH4 )2 [TiCl6 ]
(c) VOSO4
(d) K3[Cu(CN)4 ]
Answer
C
Question. Which of the following is not a characteristic of transition elements ?
(a) Variable oxidation states
(b) Formation of coloured compounds
(c) Formation of interstitial compounds
(d) Natural radioactivity
Answer
D
Question. What is the oxidation state of iron in Mohr’s salt?
(a) + 3
(b) 0
(c) + 2
(d) + 1
Answer
C
Question. Which of the following compounds volatilises on heating?
(a) MgCI2
(b) HgCI2
(c) CaCI2
(d) FeCI3
Answer
B
Question. MnO–4 reacts with bromide ion in alkaline medium to give
(a) MnBr4
(b) MnOBr2
(c) MnO2, BrO–3
(d) MnO, BrO
Answer
C
Question. Atomic radius of Ti, Zr and Hf vary
(a) Ti > Zr > Hf
(b) Ti < Zr < Hf
(c) Ti < Hf < Zr
(d) Ti < Zr = HF
Answer
D
Question. The correct statement is
(a) the earlier members of lanthanoid series resemble calcium in their chemical properties
(b) the extent of actinoid contraction is almost the same as lanthanoid contraction
(c) in general, lanthanoid and actinoids do not show variable oxidation states
(d) Ce4+ in aqueous solution is not known
Answer
A
Question. In context with the transition elements, which of the following statement is incorrect ?
(a) In addition to the normal oxidation state, the zero oxidation state is also shown by these elements in complexes
(b) In the highest oxidation state, the transition metal shows basic character and form cationic complexes
(c) In the highest oxidation state of the first five transition elements (Sc to Mn), all the 4s and 4d electrons are used for boncling
(d) Once the d5 configuration is exceeded, the tendency to involve all the 3d electrons in bonding decreases
Answer
B
Question. Transition metal ions show colour because
(a) they absorb light
(b) they emit light
(c) they are paramagnetic
(d) they exhibit d -d transition
Answer
D
Question. The magnetic moment of a salt containing Zn2+ ion is
(a) 0
(b) 1.87
(c) 5.92
(d) 2
Answer
A
Question. The gas obtained by reaction of K4Fe(CN)6 with cone. H2SO4 is
(a) H2S
(b) CO
(c) NO2
(d) CO2
Answer
B
Question. Formation of coloured ions by transition metals signifies
(a) absorption of light from UV range
(b) emission of light
(c) presence of unpaired electrons in sand p-orbitals
(d) complimentary colours to the absorbed light
Answer
D
Question. When hydrogen peroxide is added to acidified potassium dichromate, a blue colour is produced due to formation of
(a) CrO3
(b) Cr2O3
(c) CrO5
(d) CrO42-
(e) Cr2O72-
Answer
C
Question. Magnetic moment of manganese in (NH4)2 MnBr2 is
(a) 3.87 BM
(b) 5.91 BM
(c) 4.89 BM
(d) 2.82 BM
Answer
B
Question. Corrosive sublimate (HgCI2 ) can be used to distinguish between
(a) formic acid and acetic acid
(b) acetaldehyde and butanone
(c) formaldehyde and propanone
(d) All of the above
Answer
A
Question. KMnO4 (acidic/alkaline) is not decolourised by
(a) Mohr salt
(b) oxalic acid
(c) benzene
(d) propene
Answer
C
Question. The highest oxidation state exhibited by transition metals is
(a) + 7
(b) + 8
(c) + 6
(d) + 5
Answer
B
Question. Which one of the following statements is not true with regard to transition elements ?
(a) They readily form complex compounds
(b) They show variable oxidation states
(c) All their ions are colourless
(d) Their ions contain partially filled d-electrons
Answer
C
Question. Mone! metal is an alloy of
(a) Cu, Ni, Fe, Mn
(b) Cu, Sn, Zn
(c) Cu, Sn, P
(d) Cu, Zn
Answer
A
Question. Which metal gives hydrogen gas on heating with hot concentrated alkali ?
(a) Ag
(b) Ni
(c) Zn
(d) Cu
Answer
C
Question. The highest magnetic moment is shown by the transition metal ion with the configuration
(a) 3d2
(b) 3d5
(c) 3d7
(d) 3d9
Answer
B
Question. Hair dyes contain
(a) coppernitrate
(b) gold chloride
(c) silver nitrate
(d) copper sulphate
Answer
C
Question. Which of the oxide of manganese is amphoteric?
(a) MnO2
(b) Mn2O3
(c) Mn2O7
(d) MnO
Answer
A
Question. A transition metal ion exists in its highest oxidation state. It is expected to behave as
(a) a chelating agent
(b) a central metal in a coordination compound
(c) an oxidising agent
(d) a reducing agent
Answer
C
Question. Which of the following compound is expected to be coloured ?
(a) Ag2SO4
(b) CuF2
(c) MgF2
(d) CuCl
Answer
B
Question. Which one of the following reactions will occur on heatmg AgNO3 above its melting point?
(a) 2AgNO3 → 2Ag + 2NO2 + O2
(b) 2AgNO3 → 2Ag + N2 + 3O2
(c) 2AgNO3 → 2AgNO2 + O2
(d) 2AgNO3 → 2Ag + 2NO + 2O2
(e) 2AgNO3 → Ag2O + N2O3 + O2
Answer
C
Question. Pick out the correct statements from the following.
I. Cobalt (III) is more stable in octahedral complexes.
II. Zinc forms coloured ions or complexes.
III. Most of the d-block elements and their compounds are ferromagnetic.
IV. Osmium shows (VIII) oxidation state.
V. Cobalt (II) is more stable in octahedral complexes.
(a) I and II
(b) I and III
(c) II and IV
(d) I and IV
(e) II and V
Answer
D
Question. Which of the following ions has a magnetic moment of 5.93 BM ?
(Atomic number V = 23, Cr= 24, Mn= 25, Fe = 26)
(a) Mn2+
(b) Fe2+
(c) Cr2+
(d) y3+
(e) Cr3+
Answer
A
Question. Which of the following is used as purgative?
(a) HgS
(b) Hg2Cl2
(c) HgCl2
(d) ZnSO4
Answer
B
Question. Which of the following is not a member of 3d-transition series ?
(a) Fe
(c) Au
(b) Co
(d) Cu
Answer
C
Question. Which of the following transition metal ions will have definite value of magnetic moment ?
(a) Sc3+
(b) Ti3+
(c) Cu3+
(d) Zn2+
Answer
B
Question. Which of the following group of transition metals is called coinage metals ?
(a) Cu, Ag, Au
(b) Ru, Rh, Pd
(c) Fe, Co, Ni
(d) Os, Ir, Pt
Answer
A
Question. Which of the following types of metals form the most efficient catalysts ?
(a) Alkali metals
(b) Alkaline earth metals
(c) Transition metals
(d) All of these
Answer
C
Question. The magnetic moment of Cu2+ ion is
(a) 2.73
(b) zero
(c) 1.93
(d) 1.73
Answer
D
Question. The ‘spin-only’ magnetic moment [in units of Bohr magneton, (μβ )] of Ni2+ in aqueous solution would be (Atomic number of Ni = 28)
(a) 2.84
(b) 4.90
(c) 0
(d) 1.73
Answer
A
Question. Bronze is a mixture of
(a) Pb + Sn
(b) Cu + Sn
(c) Cu + Zn
(d) Pb + Zn
Answer
B
Question. Effective magnetic moment of Sc3+ ion is
(a) 1.73
(b) 0
(c) 5.92
(d) 2.83
(e) 3.87
Answer
B
Question. Which of the following pair of transition metal ions, have the same calculated values of magnetic moment?
(a) Ti2+ and V2+
(b) Fe2+ and Cu2+
(c) Cr2+ and Fe2+
(d) Co2+ and Ti2+
Answer
C
Question. Excess of KI reacts with CuSO4 solution and then Na2S2O3 solution is added to it. Which of the statements is incorrect for this reaction ?
(a) Cu2I2 fonned
(b) Cul2 is formed
(c) Na Na2S2O3 is oxidised
(d) Evolved I2 is reduced
Answer
B
Question. Which of the following is philosopher’s wool?
(a) ZnO
(b) HgO
(c) Ag2O
(d) CuO
Answer
A
Question. Ferrous sulphate (FeSO4 · 7H2O) is known as
(a) vermillion
(b) Glauber’s salt
(c) green vitriol
(d) Mohr’s salt
Answer
C
Question. In photography we use
(a) Agl
(b) NH3
(c) AgCl
(d) AgBr
Answer
D
Question. Cl2 + HgO → ?
(a) Cl2O + HgCl
(c) ClO + HgCl
(b) Cl2O + HgCl2
(d) ClO + HgCl2
Answer
B
Question. When oxyhaemoglobin changes to deoxyhaemoglobin, Fe2+ ion changes from
(a) diamagnetic to paramagnetic
(b) paramagnetic to diamagnetic
(c) diamagnetic to ferromagnetic
(d) paramagnetic to ferromagnetic
Answer
A
Question. The magnetic moment of a transition metal ion is 3 .87 BM. The number of unpaired electrons present in it is
(a) 2
(b) 3
(c) 4
(d) 5
Answer
B
Question. Potassium permanganate acts as an oxidant in alkaline and acidic medium. The final products formed from KMnO4 in the two conditions are respectively
(a) MnO2- and Mn3+
(b) Mn3+ and Mn2+
(c) Mn2+ and Mn3+
(d) MnO2 and Mn2-
(e) Mn2+, MnO2
Answer
D
Question. Mohr salt is made up of which combination of salt ?
(a) Ammonium sulphate and potash
(b) Ammonium sulphate and fe1Tous sulphate
(c) Ammonium sulphate and copper sulphate
(d) Ammonium sulphate and magnesium sulphate
Answer
B
Question. Gun metal is
(a) Cu + Zn
(b) Cu + Sn + Zn
(c) Cu + Sn
(d) Zn + Sn
Answer
B
Question. The atomic numbers of vanadium (V), chromium (Cr), manganese (Mn) and iron (Fe) are respectively 23, 24, 25 and 26. Which one of these may be expected to have the highest second ionisation enthalpy ?
(a) V
(b) Cr
(c) Mn
(d) Fe
Answer
B
Question. Formation of coloured solution is possible when metal ion in the compound contains
(a) paired electrons
(b) lone pair of electrons
(c) unpaired electrons
(d) None of these
Answer
C
Question. Which of the following pairs of elements cannot form an alloy ?
(a) Zn, Cu
(b) Fe, Hg
(c) Fe, C
(d) Hg, Na
Answer
B
Question. Match the following Columns.
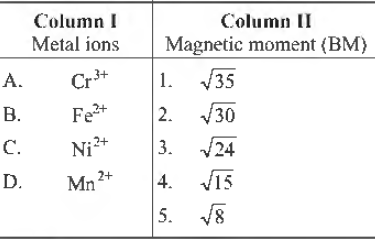
Codes
A B C D
(a) 1 3 5 4
(b) 2 3 5 1
(c) 4 3 5 1
(d) 4 5 3 1
(e) 5 1 2 3
Answer
C