Please refer to Coordination Compounds MCQ Questions Class 12 Chemistry below. These MCQ questions for Class 12 Chemistry with answers have been designed as per the latest NCERT, CBSE books and syllabus issued for the current academic year. These objective questions for Coordination Compounds will help you to prepare for the exams and get more marks.
Coordination Compounds MCQ Questions Class 12 Chemistry
Please see solved MCQ Questions for Coordination Compounds in Class 12 Chemistry. All questions and answers have been prepared by expert faculty of standard 12 based on latest examination guidelines.
Question. The IUPAC name of K4[Ni(CN)4 ] is
(a) tetrapotassium tetracyanonickelate (II)
(b) potassium tetracyanonickel (II)
(c) potassium tetracyanonickelate (0)
(d) potassium tetracyanonickelate (II)
Answer
C
Question. The lUPAC name of the complex [Co(NH3 )4 Cl2 ]Cl is
(a) dichlorotetraamminecobalt (III) chloride
(b) tetraamminedichlorocobalt (III) chloride
(c) tetraamminedichlorocobalt (II) chloride
(d) tetraamminedichlorocobalt (IV) chloride
Answer
B
Question. In which year, IUPAC draft recommends that anionic ligands will end with iodo so that chloro would become chiorido?
(a) 1994
(b) 1984
(c) 2000
(d) 2004
Answer
D
Question. A complex compound of Co3+ with molecular formula CoClx · yNH3 gives a total of 3 ions when dissolved in water. How many Cl– ions satisfy both primary and secondary valencies in this complex?
(a) 3
(b) 1
(c) 4
(d) zero
Answer
B
Question. The IUP AC name of the complex ion formed when gold dissolves in aqua-regia is
(a) tetrachloridoaurate (III)
(b) tetrachloridoaurate (I)
(c) tetrachloridoaurate (II)
(d) dichloridoaurate (III)
Answer
A
Question. According to IUP AC system, what is the correct name of the compound [Cr(NH3)3 (H2O)3 Cl3 ]
(a) Ttriamminetriaquachromium (III) chloride
(b) Triamminetriaquachromiumchloride (III)
(c) Tetraammoniumtriaquachromium (III) chloride
(d) None of the above
Answer
A
Question. lUPAC name of K3Fe(CN)6 is
(a) potassium ferricyanide
(b) hexacyano ferrate (III)
(c) potassium hexacyano ferrate (III)
(d) potassium hexacyano ferrate (II)
Answer
C
Question. Which of the following is correct lUPAC name for K2 [Cr(CN)2O2(O)2 NH3]?
(a) Potassium amrninecyanoperoxodioxochromatic (III)
(b) Potassium amminecyanoperoxodioxochromium (II)
(c) Potassium amrninecyanoperoxodioxochromium (VI)
(d) Potassium amminedicyanodioxoperoxochromate (VI)
Answer
D
Question. Ethylene diarnine is an example of
(a) monodentate ligand
(b) bidentate ligand
(c) tridentate ligand
(d) polydentate ligand
Answer
B
Question. One mole of the complex compound Co(NH3 )5 Cl3 gives 3 moles of ions on dissolution in water. One mole of the same complex reacts with two moles of AgNO3 solution to yield two moles of AgCl. The structure of the complex is
(a)[Co(NH3)4 Cl]Cl2 • NH3
(b) [Co(NH3)4 Cl2 ]Cl· NH3
(c) [Co(NH3)3Cl3 ] 2NH3
(d) [Co(NH3)5 Cl]Cl2
Answer
D
Question. The oxidation number of cobalt in K[Co(CO)4] is
(a) + 1
(b) + 3
(c) – 1
(d) – 3
Answer
C
Question. Match each coordination compound in Column I with an appropriate pair of characteristics from Column II and select the correct answer using the code given below the columns. (en = H2NCH2CH2NH2; atomic numbers : Ti = 22; Cr= 24; Co= 27; Pt = 78)
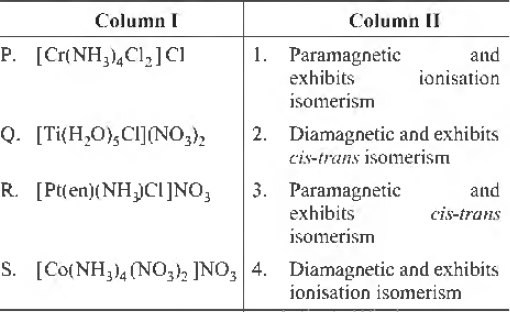
Codes
A B C D
(a) 4 2 3 1
(b) 3 1 4 2
(c) 2 1 3 4
(d) 1 3 4 2
Answer
B
Question. The volume (in mL) of 0.1 M AgNO3 required for complete precipitation of chloride ions present in 30 mL of O.Ql M solution of[Cr(H2O)5 Cl] Cl2 , as silver chloride is close to
(a) 3
(b) 4
(c) 5
(d) 6
Answer
D
Question. The IUPAC name of [Ni(PPh3 )2 Cl2 ]2+ is
(a) bisdichloro(triphenylphosphine )nickel (II)
(b) dichlorobis(triphenylphosphine )nickel (II)
(c) dichlorotriphenylphosphinenickel(II)
(d) triphenylphosphinenickel (II) dichloride
Answer
B
Question. The existence of two different coloured complexes of [Co(NH3)4Cl2 ]+ is due to
(a) ionisation isomerism
(b) coordination isomerism
(c) linkage isomerism
(d) geometrical isomerism
Answer
D
Question. Pick out the complex compound in which the central metal atom obeys EAN mle strictly.
(a) K4[Fe(CN)6]
(b) K3[Fe(CN)6 ]
(c)[Cr(H2O)6 ]Cl3
(d)[Cu(NH3)4] SO4
Answer
A
Question. The pair of [PtCl2 (NH3)4 ] Br2 and [PtBr2 (NH3)4 ]Cl2 constitutes a pair of
(a) coordination isomers
(b) linkage isomers
(c) ionisation isomers
(d) optical isomers
Answer
C
Question. Which among the following will be named as dibromidobis ( ethylenediamine) chromium (III) bromide ?
(a) [Cr(en)3 ] Br3
(b) [Cr(en)2Br2 ]Br
(c) [Cr(en)Br4 ]–
(d) [Cr(en)Br2 ]Br
Answer
B
Question. Which of the following coordination compounds would exhibit optical isomerism ?
(a) Pentaamminenitrocobalt (ill) iodide
(b) Diamminedinitroplatinum (II)
(c) Trans-dicyanobis ( ethy lenediamine)
(d) Trans-(ethylenediamine) cobalt (III) bromide
Answer
D
Question. Which one of the following complex ions has geometrical isomers ?
(a) [Co(en)3 ]3+
(b) [Ni(NH3 )5 Br]+
(c) [Co (NH3 )2 (en)2 ]3+
(d) [Cr(NH3 )4 ( en)]3+
Answer
C
Question. The IUPAC name of[Co(NH3 )6 ] [Cr(C2O4)3] is
(a) hexaaminecobalt (III) tris (oxalato) chromium
(b) hexaaminecobalt (III) tris ( oxalato) chromate (III)
(c) hexaaminecobalt tris ( oxalato )chromium (III)
(d) hexaaminecobalt (III) chromium (III) oxalate
Answer
B
Question. Benzoylacetonato beryllium exhibit isomerism of the type
(a) structural
(b) geometrical
(c) optical
(d) conformational
Answer
C
Question. Ammonia gas does not evolve from the complex FeCI3 · 4NH3 but is gives white precipitate with aqueous solution of AgNO3 . Coordination number of central metal ion in above complex is six. Give lUPAC name of the complex.
(a) ammoniumtrichlorotriammineferrum (III)
(b) tetraammineroferrum (III) chloride
(c) dichlorotetraammineferrate (II) chloride
(d) dichlorotetraammineferrum (III) chloride
Answer
B
Question. The pair(s) of coordination complexes/ions exhibiting the same kind of isomerism is (are)
(a) [Cr(NH3)5 Cl] Cl2 and [Cr(NH3 )4 Cl2 ]Cl
(b) [Co(NH3 )4 Cl2 ]+ and [Pt(NH3 )2 (H2O)Cl]+
(c) [CoBr2Cl2 ]2- and [PtBr2 Cl2 ]2-
(d) [Pt(NH3 )3 (NO3 )] Cl and [Pt(NH3 )3 Cl] Br
Answer
D
Question. Which of the following will not form optical isomers ?
(a) [Co(en)3 ]3+
(b) [Co(NH3)3 (NO2)3]
(c) [Pt(en)2 Cl2 ]2+
(d) [CrCI2 (ox)2 ]3-
Answer
B
Question. Which of the following complex species is not expected to exhibit optical isomerism ?
(a) [Co(en)3 ]3+
(b) [Co(en)2 Cl2 ]+
(c) [Co(NH3 )3 Cl3 ]
(d) [Co(en)(NH3 )Cl2]
Answer
C
Question. The ionisation isome rof [Cr(H2O)4 Cl(NO2)] a is
(a) [Cr(H2O)4 (O2N)] Cl2
(b) [Cr(H2O)4 Cl2 ](NO2)
(c) [Cr(H2O)4 Cl(ONO)]Cl
(d) [Cr(H2O)4 Cl2 (NO2 )]· H2O
Answer
B
Question. Total number of geometrical isomers for the complex [RhCl(CO)(PPh3)(NH2)] is
(a) 1
(b) 2
(c) 3
(d) 4
Answer
C
Question. Octahedral complex is
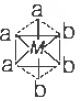
(a) cis
(b) trans
(c) mer
(d) fac
Answer
D
Question. Excess of silver nitrate solution is added to 100 mL of 0.01 M pentaaquachlorochromium (III) chloride solution. The mass of silver chloride obtained in grams is
(atomic mass of silver is 108).
(a) 287 x 10-3
(b) 143.5 x 10-3
(c) 143.5 x 10-2
(d) 287 x 10-2
Answer
A
Question. Which of the following can exhibit geometrical isomerism?
(a) [MnBr4 ]2-
(b) [Pt(NH3)3 Cl]+
(c) [PtCl2 · P(C2H5 )3]2
(d) [Fe(H2O)5 NO]2+
Answer
C
Question. Fae-mer isomerism is associated with which one of the following complexes? [M = central metal]
(a) [M (AA )2 ]
(b) [M A3B3 ]
(c) [M (AA )3 ]
(d) [M ABCD]
Answer
B
Question. Which of the following pairs represents linkage isomers ?
(a) [Cu(NH3 )4 ][PtCl4 ] and [Pt(NH3 )4 ][CuCl4 ]
(b) [Pd(PPh3 )2(NCS)2 ] and [Pd(PPh3 )2 (SCN)2 ]
(c) [Co(NH3)5 ]NO3SO4 and [Co(NH3)5 SO4 ]NO3
(d) [PtCl2 (NH3 )4 ]Br2 and [PtBr2 (NH3 )4 ]Cl2
Answer
B
Question. Which of the following can participate in linkage isomerism?
(a) NH3
(b) H2O
(c) H2NCH2CH2NH2
(d) NO–2
Answer
D
Question. For square planar complex of platinum (II), [Pt(NH3 )(Br)(Cl)Py ]2+, how many isomeric forms are possible ?
(a) Two
(b) Three
(c) Four
(d) Six
Answer
B
Question. Which of the following has on optical isomer ?
(a) [Co(NH3)3 CI]+
(b) [Co(en)(NH3 )2]2+
(c) [Co(H2O)4 (en)]3+
(d) [Co( en)2(NH3 )2 ]3+
Answer
D
Question. [Co(NH3 )5 SO4 ]Br and [Co(NH3 )5 Br]SO4 are a pair of ………. isomers.
(a) ionisation
(b) ligand
(c) coordination
(d) hydrate
Answer
A
Question. Which of the following 0.1 M complex compound solutions will have the minimum electrical conductivity?
(a) Hexammineplatinum (IV) chloride
(b) Chloropentaammineplatinum (IV) chloride
(c) Dichlorotetrammineplatinum (IV) chloride
(d) Trichlorotriarnmineplatinum (IV) chloride
Answer
D
Question. The IUPAC name of [Ni(NH3 )4 ][NiCl4 ] is
(a) tetrachloronickel (II) – tetraamminenickel (II)
(b) tetraamminenickel (II) – tetrachloronickel (II)
(c) tetraamminenickel (II) – tetrachloronickelate (II)
(d) tetrachloronickel (II) – tetraammine nickelate (0)
Answer
C
Question. A solution containing 2.675 g of CoCI3 · 6NH3 (molarmass = 267 .5 g mol-1) is passed through a cation exchanger. The chloride ions obtained in solution were treated with excess of AgNO3 to give 4.78 g of AgCl (molar mass = 143.5 g mol-1 ) . The formula of the complex is (Atomic mass of Ag= 108 u)
(a) [Co(NH3 )6 ]Cl3
(b) [CoCI2 (NH)3 ]4 Cl
(c)[CoCI3(NH3)3 ]
(d) [CoCl(NH3)5 ]Cl2
Answer
A
Question. The number of water molecule(s) directly bonded to the metal centre in CuSO4 · 5H2O is
(a) 1
(b) 2
(c) 3
(d) 4
Answer
D
Question. For the square planar complex [Mabcd]
(where, M = central metal and a, b, c and d are monodentate ligands), the number of possible geometrical isomers are
(a) 1
(b) 2
(c) 3
(d) 4
Answer
C
Question. The IUPAC name of K2[Ni(CN)4 ] is
(a) potassium tetracyanonickelate (II)
(b) potassium tetracyanatonickelate (III)
(c) potassium tetracyanatonickel (II)
(d) potassium tetracyanonickel (III)
Answer
A
Question. The number of ions fonned when hexaminecopper (II) sulphate is dissolved in water is
(a) 1
(b) 2
(c) 4
(d) 6
Answer
B
Question. The colour in the coordination compounds can be readily explained in terms of
(a) spectrochemistry
(b) chelate effect
(c) crystal field theory
(d) None of these
Answer
C
Question. Which of the following is correct statement?
(a) [Co(NH3 )6 ]2+ is paramagnetic
(b) [MnBr4 ]2- is tetrahedral
(c) [CoBr2 (en)2]– exhibits linkage isomerism
(d) [Ni(NH3 )6 ]2+ is an inner orbital complex
Answer
A.B
Question. The IUPAC name of compound K3 [Fe(CN)5 NO] is
(a) pentacyano nitrosyl potassium ferrate (II)
(b) potassium cyano pentanitrosyl ferrate (II)
(c) potassium pentacyanonitrosyl ferrate (III)
(d) potassium pentacyanonitrosyl ferrate (II)
Answer
D
Question. What is the IUPAC name of Na2[Fe(CN)5 NO]?
(a) Pentacyanonitroso sodium ferrate
(b) Pentacyanonitroso sodium ferrate (II)
(c) Sodium pentacyanonitroso ferrate (II)
(d) Sodium pentacyanonitroso ferrate
Answer
C
Question. Which kind of isomerism is exhibited by octahedral Co(NH3 )4 Br2Cl?
(a) Geometrical and ionisation
(b) Geometrical and optical
(c) Optical and ionisation
(d) Geometrical only
Answer
A
Question. The IUPAC name of the given compound [Co(NH3 )5 Cl]Cl2 is
(a) pentaarninocobaltchloridechlorate
(b) cobaltpentaamminechlorochloride
(c) pentarnmine chloro cobalt (III) chloride
(d) pen ta amino cobalt (III) chlorate
Answer
C
Question. Which one of the following has an optical isomer ?
(en= ethylenediamine)
(a) [Zn(en)(NH3 )2 ]2+
(b) [Co(en)3 ]3+
(c) [Co(H2O)4 ( en)]3+
(d) [Zn(en)2 ]2+
Answer
B
Question. According to IUP AC nomenclature sodium nitroprusside is named as
(a) sodiumpentacyanonitrosylferrate (II)
(b) sodiumpentacyanonitrosylferrate (III)
(c) sodiumnitroferricyanide
(d) sodiumnitroferrocyanide
Answer
B
Question. Which one amongst the following, exhibit geometrical isomerism ?
(a) [CoIII (NH3 )5 Br]SO4
(b) CoIII [EDTA]1-
(c) [CrCoIII (SCN)6 ]3-
(d) [PtII (NH3 )2 Cl2]
Answer
D
Question. The ratio of magnetic moment (spin only value) between [FeF6 ]3- and[Fe(CN)6 ]3- is approximately
(a) 4
(b) 2
(c) 5
(d) 3
Answer
D
Question. The coordination compounds,
[Co(NH3 )6 ]3+ [Cr(CN)6 ]3-
and [Cr(NH3 )6 ]3+[Co(CN)6 ]3- are the examples of
(a) linkage isomerism
(b) coordination isomerism
(c) ionisation isomerism
(d) geometrical isomerism
Answer
B
Question. Type of isomerism shown by [Cr(NH3 )5 NO2 ]Cl2 is
(a) optical
(b) ionisation
(c) geometrical
(d) linkage
Answer
D
Question. IUPAC name of Na3 [Co(NO2 )6 ] is
(a) sodium hexanitrito cobaltate (II)
(b) sodium hexanitro cobaltate (III)
(c) sodium hexanitrito cobaltate (III)
(d) sodium cobaltinitrite (II)
Answer
B
Question. Which is not true of the coordination compound [Co( en)2 Cl2 ]Cl ?
(a) Exhibits geometrical isomerism
(b) Exhibits optical isomerism
(c) Exhibits ionisation isomerism
(d) Is an octahedral complex
(e) Is a cationic complex
Answer
C
Question. Both Co3+ and Pt4+ have a coordination number of six. Which of the following pairs of complexes will show approximately the same electrical conductance for their 0.001 M aqueous solutions?
(a) CoCI3 · 4NH3 and PtCI4 · 4NH3
(b) CoCI3 · 3NH3 and PtCI4 · 5NH3
(c) CoCI3 · 6NH3 and PtCI4 · 5NH3
(d) CoCI3 · 6NH3 and PtCI4 · 3NH3
Answer
C
Question. The spin only magnetic moment value (in BM unit) of Cr(CO)6 is
(a) zero
(b) 2.84
(c) 4.90
(d) 5.92
Answer
A
Question. [Fe(NO2)3 Cl3 ] and [Fe(O—NO)3 Cl3 ] shows
(a) linkage isomerism
(b) geometrical isomerism
(c) optical isomerism
(d) None of the above
Answer
A
Question. Match the following columns.
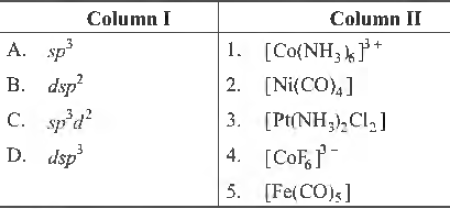
A B C D
(a) 5 2 4 3
(b) 2 3 4 5
(c) 2 3 1 5
(d) 3 2 4 1
Answer
B
Question. Among [Fe(H2O)6]3+, [Fe(CN)6]3- , species, the hybridisation state of the Fe atom are, respectively
(a) d2 sp3,d2 sp3 , sp3d2
(b) sp3d2 ,d2 sp3 ,d2 sp3
(c) sp3d2 ,d2 sp3 ,sp3d2
(d) None of the above
Answer
C
Question. The anti-cancer drug cis-platin has the formula Pt(NH3)2Cl2 . There is another isomer, trans-platin, that is not medically active. What is the shape of cis-platin ?
(a) Tetrahedral
(b) Octahedral
(c) Square planar
(d) Trigonal bipyramidal
Answer
C
Question. Th IUPAC name of the compound [CuCl2 (CH3NH2)2] is
(a) dichloro bis (dimethylamine) copper (II)
(b) dichloro bis (methylamine) copper (II)
(c) dimethylaminecopper (II) chloride
(d) bis(dimethylamine) copper (II) chloride
Answer
B
Question. The question when worked out will result in one integer from Oto 9 (both inclusive).
A list of species having the fommla XZ4 is given below
XeF4 , SF4 ,SiF4 , BF4, BrF4, [Cu(NH3 )4 ]2+
[FeCl4 ]2- , [CoCl4]2- and [PtCl4 ]2-
Defining shape on the basis of the location of X and Z atoms, the total number of species having a square planar shape is
(a) 3
(b) 2
(c) 4
(d) 6
Answer
C
Question. For the given complex [CoCl2 ( en)(NH3 )2]+ , the number of geomettical isomers, the number of optical isomers and total number of isomers of all type possible respectively are
(a) 2, 2 and 4
(b) 2, 1 and 3
(c) 2, 0 and 2
(d) 0, 2 and 2
Answer
B
Question. Which of the following compounds shows optical isomerism?
(a) [Co(CN)6 J3-
(b) [Cr(C2O4)3 ]3-
(c) [ZnCl4 ]2-
(d) [Cu(NH3 )4 ]2+
Answer
B
Question. Give name of the complex, name should specify the position of ligands.

(a) bistransphosphinecarbonylchloroiridium [II]
(b) carbonylchlorobistransphosphineiridium [III]
(c) carbonylchlorobistransphosphineiridium [I]
(d) chlorocarbony lbistransphosphineiridium [I]
Answer
C
Question. The IUPAC name of[Co(NH3 )5ONO]2+ ion is
(a) pentaamminenitritocobalt (IV) ion
(b) pentaamminenitrocobalt (II) ion
(c) pentaamminenitritocobalt (III) ion
(d) pentaamminenitrocobalt (V) ion
Answer
C
Question. Which one of the following octahedral complexes will not show geometrical isomerism? (A and Bare monodentate ligands)
(a) [MA4B2 ]
(b) [MA5B]
(c) [MA2B4 ]
(d) [MA3B3 ]
Answer
B
Question. The IUP AC name for the complex
[Co(NO2)(NH3 )5 ]Cl2 is
(a) nitrito-N-pentamn1inecobalt (III) chloride
(b) nitrito-N-pentamminecobalt (II) chloride
(c) pentaamminenitrito-N-cobalt (II) chloride
(d) pentaamminenitrito-N-cobalt (III) chloride
Answer
D


