Please refer to Kinetic Theory MCQ Questions Class 11 Physics below. These MCQ questions for Class 11 Physics with answers have been designed as per the latest NCERT, CBSE books, and syllabus issued for the current academic year. These objective questions for Kinetic Theory will help you to prepare for the exams and get more marks.
Kinetic Theory MCQ Questions Class 11 Physics
Please see solved MCQ Questions for Kinetic Theory in Class 11 Physics. All questions and answers have been prepared by expert faculty of standard 11 based on the latest examination guidelines.
MCQ Questions Class 11 Physics Kinetic Theory
Question. The kinetic theory of gases
(a) explains the behaviour of an ideal gas.
(b) describes the motion of a single atom or molecule.
(c) relates the temperature of the gas with K.E. of atoms of the gas
(d) All of the above
Answer
D
Question. If pressure of a gas contained in a closed vessel is increased by 0.4% when heated by 1ºC, the initial temperature must be
(a) 250 K
(b) 250ºC
(c) 2500 K
(d) 25ºC
Answer
A
Question. The value of critical temperature in terms of van der Waal’s constant a and b is given by
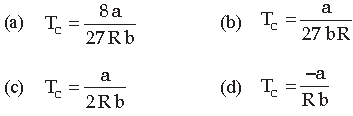
Answer
A
Question. A fixed mass of gas at constant pressure occupies a volume V. The gas undergoes a rise in temperature so that the root mean square velocity of its molecules is doubled. The new volume will be
(a) V/2
(b) V / √2
(c) 2 V
(d) 4 V
Answer
D
Question. Two gases A and B having the same temperature T, same pressure P and same volume V are mixed. If the mixture is at the same temperature T and occupies a volume V, the pressure of the mixture is
(a) 2 P
(b) P
(c) P/2
(d) 4 P
Answer
A
Question. Four molecules have speeds 2 km/sec, 3 km/sec, 4 km/sec and 5 km/sec. The root mean square speed of these molecules (in km/sec) is
(a) √54 / 4
(b) √54 / 2
(c) 3.5
(d) 3√3
Answer
A
Question. In kinetic theory of gases, it is assumed that molecules
(a) have same mass but can have different volume
(b) have same volume but mass can be different
(c) have different mass as well as volume
(d) have same mass but negligible volume.
Answer
D
Question. N molecules, each of mass m, of gas A and 2 N molecules, each of mass 2 m, of gas B are contained in the same vessel which is maintained at a temperature T. The mean square of the velocity of molecules of B type is denoted by v2 and the mean square of the X component of the velocity of A type is dentoed by ϖ2, ϖ2/v2 is
(a) 2
(b) 1
(c) 1/3
(d) 2/3
Answer
D
Question. The adjoining figure shows graph of pressure and volume of a gas at two tempertures T1 and T2. Which of the following inferences is correct?
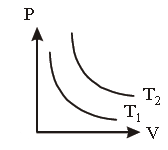
(a) T1 > T2
(b) T1 = T2
(c) T1 < T2
(d) None of these
Answer
A
Question. One litre of oxygen at a pressure of 1 atm, and 2 litres of nitrogen at a pressure of 0.5 atm. are introduced in the vessel of 1 litre capacity, without any change in temperature. The total pressure would be
(a) 1.5 atm.
(b) 0.5 atm.
(c) 2.0 atm.
(d) 1.0 atm.
Answer
C
Question. The correct statement of the law of equipartition of energy is
(a) the total energy of a gas is equally divided among all the molecules.
(b) The gas possess equal energies in all the three directions x,y and z-axis.
(c) the total energy of a gas is equally divided between kinetic and potential energies.
(d) the total kinetic energy of a gas molecules is equally divided among translational and rotational kinetic energies.
Answer
B
Question. A sample of an ideal gas occupies a volume of V at a pressure P and absolute temperature. T. The mass of each molecule is m. The equation for density is
(a) m k T
(b) P/k T
(c) P/(k T V)
(d) P m/k T
Answer
D
Question. The internal energy of an ideal gas is
(a) the sum of total kinetic and potential energies.
(b) the total translational kinetic energy.
(c) the total kinetic energy of randomly moving molecules.
(d) the total kinetic energy of gas molecules.
Answer
D
Question. The air in a room has 15 gm of water vapours per cubic metre of its volume. However for saturation one cubic metre of volume requires 20 gm of water vapour then relative humidity is
(a) 50%
(b) 75%
(c) 20%
(d) 25%
Answer
B
Question. The root mean square speed of the molecules of a diatomic gas is v. When the temperature is doubled, the molecules dissociate into two atoms. The new root mean square speed of the atom is
(a) √2v
(b) v
(c) 2v
(d) 4v
Answer
C
Question. A gas mixture consists of molecules of type 1, 2 and 3, with molar masses m1 > m2 > m3. vrms and K→ are the r.m.s. speed and average kinetic energy of the gases. Which of the following is true?
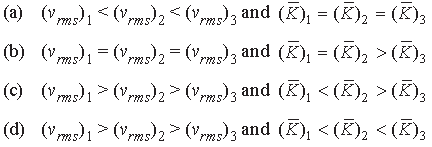
Answer
A
Question. At room temperature, the rms speed of the molecules of a certain diatomic gas is found to be 1930 m/s. The gas is
(a) H2
(b) F2
(c) O2
(d) Cl2
Answer
A
Question. Which of the following formula is wrong?

Answer
D
Question. In the kinetic theory of gases, which of these statements is/are true ?
(i) The pressure of a gas is proportional to the mean speed of the molecules.
(ii) The root mean square speed of the molecules is proportional to the pressure.
(iii) The rate of diffusion is proportional to the mean speed of the molecules.
(iv) The mean translational kinetic energy of a gas is proportional to its kelvin temperature.
(a) (ii) and (iii) only
(b) (i),(ii)and (iv) only
(c) (i) and (iii) only
(d) (iii) and (iv) only
Answer
D
Question. For a gas if ratio of specific heats at constant pressure and volume is g then value of degrees of freedom is

Answer
B
Question. The density of air at pressure of 105 Nm–2 is 1.2 kg m–3. Under these conditions, the root mean square velocity of the air molecules in ms–1 is
(a) 500
(b) 1000
(c) 1500
(d) 3000
Answer
A
Question. The perfect gas equation for 4 gram of hydrogen gas is
(a) PV = RT
(b) PV = 2RT
(c) PV= (1/2) RT
(d) PV = 4RT
Answer
B
Question. Maxwell’s laws of distribution of velocities shows that
(a) the number of molecules with most probable velocity is very large
(b) the number of molecules with most probable velocity is very small
(c) the number of molecules with most probable velocity is zero
(d) the number of molecules with most probable velocity is exactly equal to 1
Answer
A
Question. According to kinetic theory of gases, which one of the following statement(s) is/are correct?
(a) Real gas behave as ideal gas at high temperature and low pressure.
(b) Liquid state of ideal gas is impossible
(c) At any temerature and pressure, ideal gas obeys Boyle’s law and charles’ law
(d) The molecules of real gas do not exert any force on one another.
Answer
A, B, C
Question. For hydrogen gas Cp – Cv = a and for oxygen gas Cp – Cv = b. So, the relation between a and b is given by
(a) a = 16 b
(b) 16 a = b
(c) a = 4 b
(d) a = b
Answer
D
Question. The relation between the gas pressure P and average kinetic energy per unit volume E is

Answer
D
Question. The r.m.s. velocity of oxygen molecule at 16ºC is 474 m/sec. The r.m.s. velocity in m/s of hydrogen molecule at 127ºC is
(a) 1603
(b) 1896
(c) 2230.59
(d) 2730
Answer
C
Question. The total degree of freedom of a CO2 gas molecule is
(a) 3
(b) 6
(c) 5
(d) 4
Answer
Question. The gases are at absolute temperature 300ºK and 350ºK respectively. The ratio of average kinetic energy of their molecules is
(a) 7 : 6
(b) 6 : 7
(c) 36 : 49
(d) 49 : 36
Answer
B
Question. If one mole of a monatomic gas (g = 5/3) is mixed with one mole of a diatomic gas (g = 7/3), the value of g for the mixture is
(a) 1.40
(b) 1.50
(c) 1.53
(d) 3.07
Answer
C
Question. On a particular day, the relative humidity is 100% and the room temperature is 30ºC, then the dew point is
(a) 70ºC
(b) 30ºC
(d) 100ºC
(d) 0ºC
Answer
B
Question. The molecules of a given mass of gas have a root mean square velocity of 200m s–1 at 27°C and 1.0 × 105 N m–2 pressure. When the temperature is 127°C and the pressure 0.5 × 105 Nm–2, the root mean square velocity in ms–1, is
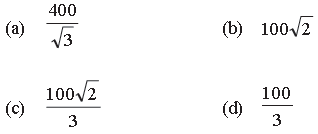
Answer
A
Question. If masses of all molecule of a gas are halved and their speed doubled then the ratio of initial and final pressure will be
(a) 2 : 1
(b) 1 : 2
(c) 4 : 1
(d) 1 : 4
Answer
B
Question. The velocity of the molecules of a gas at temperature 120 K is v. At what temperature will the velocity be 2 v?
(a) 120 K
(b) 240 K
(c) 480 K
(d) 1120 K
Answer
C
Question. The temperature of the mixture of one mole of helium and one mole of hydrogen is increased from 0°C to 100°C at constant pressure. The amount of heat delivered will be
(a) 600 cal
(b) 1200 cal
(c) 1800 cal
(d) 3600 cal
Answer
B
Question. The density of a gas is 6 × 10–2 kg/m3 and the root mean square velocity of the gas molecules is 500 m/s. The pressure exerted by the gas on the walls of the vessel is
(a) 5×103 N/m2
(b) 1.2×10–4 N/m2
(c) 0.83×10–4 N/m2
(d) 30N/m2
Answer
A
Question. Helium gas is filled in a closed vessel (having negligible thermal expansion coefficient) when it is heated from 300 K to 600 K, then average kinetic energy of helium atom will be
(a) √2 times
(b) 2 times
(c) unchanged
(d) half
Answer
B
Question. One mole of a gas occupies 22.4 lit at N.T.P. Calculate the difference between two molar specific heats of the gas. J = 4200 J/kcal.
(a) 1.979 k cal/kmol K
(b) 2.378 k cal/kmol K
(c) 4.569 kcal/kmol K
(d) 3.028 k cal/ kmol K
Answer
A
Question. How many degrees of freedom are associated with 2 grams of He at NTP ?
(a) 3
(b) 3.01 × 1023
(c) 9.03 × 1023
(d) 6
Answer
C
Question. At constant pressure, the ratio of increase in volume of an ideal gas per degree rise in kelvin temperature to its original volume is (T = absolute temperature of the gas) is
(a) T2
(b) T
(c) 1/T
(d) 1/T2
Answer
C
Question. The K.E. of one mole of an ideal gas is E = (3/2) RT. Then Cp will be
(a) 0.5 R
(b) 0.1 R
(c) 1.5 R
(d) 2.5 R
Answer
D
Question. If 2 mole of an ideal monatomic gas at temperature T0 is mixed with 4 moles of another ideal monatomic gas at temperature 2T0, then the temperature of the mixture is
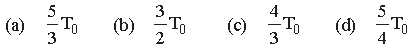
Answer
A
Question. The temperature at which the root mean square velocity of the gas molecules would become twice of its value at 0°C is
(a) 819°C
(b) 1092°C
(c) 1100°C
(d) 1400°C
Answer
A
Question. If the critical temperature of a gas is 100ºC, its Boyle temperature will be approximately
(a) 337.5ºC
(b) 500ºC
(c) 33.3ºC
(d) 1000ºC
Answer
D
Question. At what temperature is the r.m.s. velocity of a hydrogen molecule equal to that of an oxygen molecule at 47ºC
(a) 80 K
(b) –73 K
(c) 3 K
(d) 20 K
Answer
D
Question. A vessel contains air at a temperature of 15ºC and 60% R.H. What will be the R.H. if it is heated to 20ºC? (S.V.P. at 15ºC is 12.67 & at 20ºC is 17.36mm of Hg respectively)
(a) 262%
(b) 26.2%
(c) 44.5%
(d) 46.2%
Answer
C
Question. Gases exert pressure on the walls of the container because the gas molecules
(a) possess momentum
(b) collide with each other
(c) have finite volume
(d) obey gas laws.
Answer
A
Question. Let v denote the rms speed of the molecules in an ideal diatomic gas at absolute temperature T. The mass of a molecule is ‘m’ Neglecting vibrational energy terms, the false statement is
(a) a molecule can have a speed greater than √2v
(b) v is proportional to √T
(c) the average rotational K.E. of a molecule is (1/4)mv2
(d) the average K.E. of a molecule is (5/6)mv2
Answer
C
Question. To what temperature should be the hydrogen at 327°C be cooked at constant pressure so that the root mean square velocity of its molecules becomes half of its previous value
(a) –123°C
(b) 120°C
(c) –100°C
(d) 0°C
Answer
A
Question. At what temperature, pressure remaining constant, will the r.m.s. velocity of a gas be half of its value at 0ºC?
(a) 0ºC
(b) –273ºC
(c) 32ºC
(d) –204ºC
Answer
D
Question. The ratio of principal molar heat capacities of a gas is maximum for
(a) a diatomic gas
(b) a monatomic gas
(c) a polyatomic gas having linear molecules.
(d) a polyatomic gas having non-linear molecules.
Answer
B


