Please refer to Organic Chemistry – Some Basic Principles and Techniques MCQ Questions Class 11 Chemistry below. These MCQ questions for Class 11 Chemistry with answers have been designed as per the latest NCERT, CBSE books, and syllabus issued for the current academic year. These objective questions for Organic Chemistry – Some Basic Principles and Techniques will help you to prepare for the exams and get more marks.
Organic Chemistry – Some Basic Principles and Techniques MCQ Questions Class 11 Chemistry
Please see solved MCQ Questions for Organic Chemistry – Some Basic Principles and Techniques in Class 11 Chemistry. All questions and answers have been prepared by expert faculty of standard 11 based on the latest examination guidelines.
MCQ Questions Class 11 Chemistry Organic Chemistry – Some Basic Principles and Techniques
Question. The compound which contains all the four 1°, 2° ,3° and4° carbon atoms is
(a) 2, 3-dimethylpentane
(b) 3-chloro-2, 3- dimethylpentane
(c) 2, 3, 4-trimethylpentane
(d) 3, 3-dimethylpentane
Answer
B
Question. The correct IUP AC name of
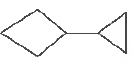
is
(a) 1-cyclopropyl cyclobutane
(b) l, 1-dicyclobutane
(c) l-cyclobutane-1-cyclopropane
(d) None of the above
Answer
A
Question. Which one of the following is not true regarding electromeric effect?
(a) It is temporary effect
(b) It operates on multiple bonds
(c) It requires on attacking reagent
(d) It results in the appearance of partial charges on the carbon atom
Answer
D
Question. Electromeric effect is
(a) permanent effect
(b) temporary effect
(c) resonance effect
(d) inductive effect
Answer
B
Question. What is the correct IUPAC name of
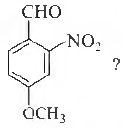
(a) 4-methox y-2-ni trobenzaldehyde
(b) 4-fonnyl-3-nitro anisole
(c) 4-methoxy-6-nitrobenzaldehyde
(d) 2-fonnyl-5-methoxy nitrobenzene
Answer
A
Question. Among the following compounds, the most acidic is
(a) p-nitrophenol
(b) p-hydroxybenzoic acid
(c) o-hydroxybenzoic acid
(d) p-toluic acid
Answer
C
Question. The IUPAC aame of
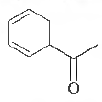
is
(a) 1-cyclohexa-2, 4-dienylethanone
(b) 3-cyclohexa-2, 4-dienylethanone
(c) 1 -cyclohexa-3, 5-dienylethanone
(d) 3-cyclohexa-3, 5-dienylethanone
Answer
A
Question. The IUPAC name of the compound (CH3 )2 CH—CH=CH—CHOH—CH3 is
(a) 5-methylhex-3-en-2-ol
(b) 2-methylhex-3-en-5-ol
(c) 2-hydroxy-5-methyl-3-hexene
(d) 5-hydroxy-2-methyl-3-hexene
Answer
A
Question. IUPAC name of CH3CH2C(Br) = CH—Cl is
(a) 2-bromo-1-chloro butene-1
(b) 1-chloro-2-bromo butene-1
(c) 3-chloro-2-bromo butene-2
(d) None of the above
Answer
A
Question. The IUPAC name of CH3—C—CH—CH3 is
ll l
O CH3
(a) 2-methyl-3-butanone
(b) 3-methyl-butan-2-one
(c) 3-methyl butanone
(d) None of the above
Answer
A
Question. The IUPAC name of the compound CH3CONHBr is
(a) 1-bromoacetamide
(b) ethanoylbromide
(c) N-bromoethanamide
(d) None of the above
Answer
C
Question. n-pentane and 2-methylbutane is a pair of
(a) enantiomers
(b) stereo isomers
(c) diastereomers
(d) constitutional isomers
Answer
D
Question. Nitroethane can exhibit which one of the following kinds of isomerism
(a) Metamerism
(b) Optical activity
(c) Tautomerism
(d) Position isomerism
Answer
C
Question. Number ofisomers possible for C4H8O is
(a) 3
(b) 4
(c) 5
(d) 6
Answer
D
Question. What kind of isomerism is possible for 1-chloro-2-nitroethene?
(a) Functional group isomerism
(b) Position isomerism
(c) EIZ isomerism
(d) Optical isomerism
Answer
C
Question. Which of the following will be chiral ?
(a) CH3CHCl2
(b) CH3CHBrCl
(c) CD2Cl2
(d) CH2ClBr
Answer
B
Question. Which of the following shows geometrical isomerism ?
(a) C2H5Br
(b) (CH2) (COOH)2
(c) (CH)2 (COOH)2
(d) C2H6
Answer
C
Question(a) 1, 1-dichloro-1-pentene
(b) 1, 2-dichloro-1-pentene
(c) 1, 3-dichloro-2-pentene
(d) 1, 4-dichloro-2-pentene
Answer
A
Question. Racemic mixture is fonned by mixing two
(a) isomeric compounds
(b) chiral compounds
(c) meso compounds
(d) enantiomers with chiral carbon
Answer
B
Question. Eno! content is highest in
(a) acetone
(b) acetophenone
(c) acetic acid
(d) acetyl acetone
Answer
D
Question. Which of the following is a pair offunctionaI isomers?
(a) CH3COCH3, CH3CHO
(b) C2H5CO2H,CH3CO2CH3
(c) C2H5CO2H, CH3CO2C2H5
(d) CH3CO2H, CH3CHO
Answer
B
Question. The epoxide ring consists of which of the following?
(a) Three membered ring with two carbon and one oxygen
(b) Four membered ring with three carbon and one oxygen
(c) Five membered ring with four carbon and one oxygen
(d) Six membered ring with five carbon and one oxygen
Answer
A
Question. IUPAC name of the following compounds is
O O
ll ll
H3C—CH2—C—H2C—CH2—C—OCH3
(a) ethyl-4-oxoheptanoate
(b) methyl-4-oxoheptanoate
(c) ethyl-4-oxohexanoate
(d) methyl 4-oxohexanoate
Answer
D
Question. The correct order of increasing basicity of the given conjugate bases (R = CH3 ) is
(a) RCOO < HC=C < R < NH2
(b) R < HC= C < RCOO< NH2
(c) RCO < NH2 < HC=C < R
(d) RCOO< HC=C< NH2 < R
Answer
A
Question. Inductive effect involves
(a) delocalisation of σ-electrons
(b) displacement of σ-electrons
(c) delocalisation of n-electrons
(d) displacement of n-electrons
Answer
C
Question. The IUPAC name of C6H5COCl is
(a) benzoyl chloride
(b) benzene chloro ketone
(c) benzene carbonyl chloride
(d) chloro phenyl ketone
Answer
A
Question. What is the formula of tertiary butyl alcohol?
(a) CH3—CH(CH3 )—CH2—OH
(b) CH3—(CH2 )2 OH
(c) CH3—CH(OH)—CH2—CH3
(d) (CH3)3 ·C—OH
Answer
D
Question. The IUPAC name of CH3CHOHCH2CH(CH3)CHO is
(a) 2-hydroxy-4-methyl-pentanal
(b) 4-hydroxy-2-methyl-pentanal
(c) 2-hydroxy-2-methyl-pentanal
(d) 2-methyl-pent-4-ol-l-al
Answer
A
Question. The IUPAC name of CH3—CH—CH= C—CHO is
l l
OH CH3
(a) 4-hydroxy-1-methylpentanal
(b) 4-hydroxy-2-methylpent-2-en-1-al
(c) 2-hydroxy-4-methylpent-3-en-5-al
(d) 2-hydroxy-3-methy lpent-2-en-5-al
Answer
A
Question. The IUP AC name of acryldehyde is
(a) prop-2-en-1-al
(b) propenyl aldehyde
(c) but-2-en-1-al
(d) propenal
Answer
A
Question. Write the IUPAC name of
CH3—O—CH—CH2—CH3
l
CH3
(a) 3-methoxybutane
(b) 2-methoxybutane
(c) 3-methyl-3-methoxypropane
(d) butoxy methane
Answer
A
Question. IUPAC name of CH3CH= C—CH3 is
I
CH2
I
CH3
(a) 2-ethyl butene
(b) 2-ethyl but-2-ene
(c) 3-methyl pent-2-ene
(d) None of these
Answer
C
Question. Formic acid is a stronger acid than acetic acid. This can be explained using
(a) + M-effect
(b) – I-effect
(c) + I-effect
(d) – M-effect
Answer
A
Question. Geometrical isomerism is reflected by which of the compounds?
(a) 3-phenyl- 1-butene
(b) 2-phenyl- 1 -butene
(c) 1, 1-diphenyl-1-propene
(d) 1-phenyl-2-butene
Answer
D
Question. The number of possible structural isomers of butene are
(a) 3
(b) 2
(c) 4
(d) 5
(e) 1
Answer
A
Question. Disymmetric object is one which is
(a) superirnposable on its mirror image
(b) non-superimposable on its mirror image
(c) optically inactive
(d) achiral
Answer
A
Question. The stability of Me2C= CH2 is more than that of Me CH2CH=CH2 due to the
(a) inductive effect of Me groups
(b) resonance effect of Me groups
(c) hyperconjugative effect of the Me group
(d) resonance as well as inductive effect of Me group
Answer
C
Question. Which of the following species does not exert a resonance effect?
(a) C6H5NH2
(b) C6H5N+H3
(c) C6H5OH
(d) C6H5Cl
Answer
B
Question. Dichloroacetic acid is a stronger acid than acetic acid. This is due to the occurrence of
(a) mesometic effect
(b) hyperconjugation
(c) inductive effect
(d) steric effect
Answer
C
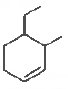
Question. The IUP AC name of the compound shown below is
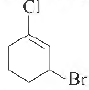
(a) 2-bromo-6-chlorocyclohex-1-ene
(b) 6-bromo-2-chlorocyclohexene
(c) 3-bromo- 1-chlorocyclohexene
(d) 1-bromo-3-chlorocyclohexene
Answer
C
Question. CH3CH2Cl undergoes homolytic fission, produces .
• •
(a) CH3CH2 and Cl
⊕
(b) CH3CH2 and Cl
⊕ •
(c) CH3CH2 and Cl
•
(b) CH3CH2 and Cl
Answer
A
Question. The reaction intermediate produced by homolytic cleavage of a bond is called
(a) carbene
(b) carbocation
(c) carbanion
(d) free radical
Answer
D
Question. Which kind of fission is favoured by sunlight?
(a) Heterolytic fission
(b) Homolytic fission
(c) Both (a) and (b)
(d) None of these
Answer
B
Question. IUPAC name of the following compound is

(a) 3, 5-dimethylcyclohexene
(b) 3, 5-dimethyl-1-cyclohexene
(c) 1, 5-dimethyl-5-cyclohexene
(d) 1, 3-dimethyl-5-cyclohexene
Answer
A
Question.

(a) 3, 3-dimethyl-1-hydroxy cyclohexane
(b) 1, 1 -dimethyl-3-hydroxy cyclohexane
(c) 3, 3-dimethy-1-cyclohexanol
(d) 1, 1-dimethyl-3-cyclohexanol
Answer
C
Question. The correct order for homolytic bond dissociation energies (ΔH in kcal/mol) for CH4 (A),C2H6 (B) and CH3Br (C), under identical experimental conditions, is
(a) C > B > A
(b) B > C > A
(c) C > A > B
(d) A > B > C
Answer
D
Question. Which of the following is possessed by a nucleophile?
(a) A lone pair of electron
(b) Positive charge
(c) Negative charge
(d) None of the above
Answer
A
Question. IUP AC name of the compound
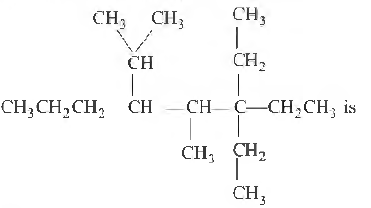
(a) 5-methyl-4-isopropyl-6, 6′-diethyloctane
(b) 3, 3-dimethyl-3-ethyl-5-isopropyloctane
(c) 3, 3-diethyl-4-methyl-5-(1, I-dimethyl) octane
(d) 3, 3-diethyl-4-methyl-5-(1′-methyl ethyl) octane
Answer
D
Question. Which of the following has most acidic hydrogen?
(a) 3-hexanone
(b) 2, 4-hexanedione
(c) 2, 4-hexanedione
(d) 2, 3-hexanedione
Answer
B
Question. Orbital interaction between the σ-bonds of a substituent group and a neighbouring n-orbital is known as
(a) hyperconjugation
(b) inductive effect
(c) steric effect
(d) electric quadrapole interaction
Answer
A
Question. Bicyclo (1, I, 0) butane is

Answer
C
Question. Which of the following resonating structures of 1-methoxy-1, 3-butadiene is least stable?
Θ ⊕
(a) CH2 — CH=CH — CH=O—CH3
(b) CH2=CH2 – CH — CH=O—CH3
Θ ⊕
(c) CH2 — CH—CH=CH—O—CH3
Θ ⊕
(d) CH2=CH—CH—CH—O—CH3
Answer
C
Question. Amongst the following, the compound that can most readily get sulphonated is
(a) benzene
(b) toluene
(c) nitrobenzene
(d) chlorobenzene
Answer
B
Question. The systematic (IUPAC) name of the compound with the following structural formula will be
Answer
D
Question. +I-effect is shown by
(a) — CH3
(b) — Br
(c) — Cl
(d) — NO2
Answer
A
Question. Chlorobenzene is o, p -directing in electrophilic substitution reaction. The directing influence is explained by
(a) + M of Ph
(b) + I of Cl
(c) + M of Cl
(d) – I of Ph
Answer
C
Question. The basicity of aniline is less than that of cyclohexylamine. This is due to
(a) +R effect of — NH2 group
(b) – I effect of — NH2 group
(c) -R effect of — NH2 group
(d) hyperconjugation effect
Answer
A
Question. A solution of (- d) 1-chloro-1-phenylelnane in toluene a racemises slowly in the presence of a small amounts of SbCI5 due to the formation of
(a) carbanion
(b) carbene
(c) carbocation
(d) free radical
Answer
C
Question. Which of the following compounds has incorrect IUPAC nomenclature?
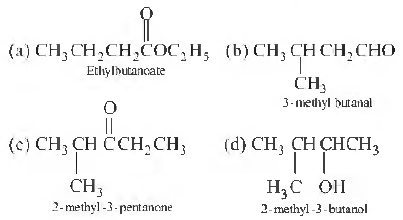
Answer
D
Question. PhCHCIBr →t BuO- carbene.
In the reaction, which of the following is most probable?
(a) : CHBr
(b) : CHCl
(c) : CPhBr
(d) : CPhCl
Answer
D
Question. Among the following carbocations
+ +
Ph2CCH2Me (I), PhCH2CH2CHPh (II),
+ +
Ph2CCH2Me (III) and Ph2C(Me )CH2 (IV), the order of stability is
(a) IV > II > I > III
(b) I > II > III > IV
(c) II > I > IV > III
(d) I > IV > III > II
Answer
B
Question. The total number of contributing structures showing hyperconjugation (involving C-H bonds) for the following carbocation is
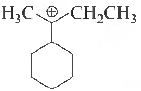
(a) three
(b) five
(c) eight
(d) six
Answer
B
Question. The highest electrical conductivity of the following aqueous solutions is of
(a) 0.1 M difluoroacetic acid
(b) 0.1 M fluoroacetic acid
(c) 0.1 M chloroacetic acid
(d) 0.1 M acetic acid
Answer
A
Question. Which compound shows geometrical isomerism among the following?
(a) CH3—C—H
ll
CH3—C—H
CH3
l
(b) HO—C—H
l
COOH
(c) CH3CH2 — CH2CH3
(b) CH3CH2COOC2CH5
Answer
A
Question. Amongst the following, the total number of compounds soluble in aqueous NaOH is

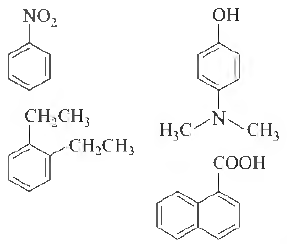
(a) 1
(b) 2
(c) 3
(d) 4
Answer
D
Question. The well known compounds (+)-lactic acid and (—) -lactic acid, have the same molecular formula, C3H6O3. The correct relationship between them is
(a) constitution isomerism
(b) geometrical isomerism
(c) identicalness
(d) optical isomerism
Answer
D
Question. The compound which gives the most stable carboniwn ionon dehydration is
(a) CH3CH(CH3 )CH2OH
(b) (CH3 )3 COH
(c) CH2 = CHCH2CH2OH
(d) CH3CHOHCH2 — CH3
Answer
B
Question. The correct acidity order of the following is
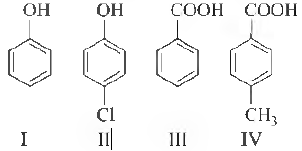
(a) III > IV > II > I
(b) IV > III > I > II
(c) III > II > I > IV
(d) II > III > IV > I
Answer
A
Question. Due to the presence of an unpaired electron, free radicals are
(a) cations
(b) anions
(c) chemically inactive
(d) chemically reactive
Answer
D
Question. Consider the following carbocations,
I. H6CH5C+H2
II. C6H5CH2C+H2
III. C6H5C+HCH3
IV. C6H5C+(H3)2
The cotTect sequence of the stability of these is
(a) II < I < III < IV
(b) II < III < I < IV
(c) III < l < II < IV
(d) IV < III < I < II
Answer
A
Question. Given,

The decreasing order of the acidic character is
(a) A>B>C
(b) B>A>C
(c) B> C> A
(d) C> B> A
Answer
C
Question. Which one of the nitrogen atoms in
O
II
H2N—NH—C—NH2 is the most nucleophilic?
I II III
(a) Only III
(b) Only I
(c) Only II
(d) All three N-atoms
Answer
B
Question. Which of the following is not true for carbanions?
(a) The carbon carrying the charge has eight valence electrons
(b) They are formed by heterolytic fission
(c) They are paramagnetic
(d) The carbon carrying the charge is sp3-hybridised
Answer
C
Question. Which one of the following carbanions is the least stable?
(a) CH3CH–2
(b) HC = C–
(c) (C6H5 )3 c–
(d) CH-3
(e) (CH3 )3 C–
Answer
E
Question. Which is the most stable carbocation?
(a) iso-propy1 cation
(b) Triphenylmethyl cation
(c) Ethyl cation
(d) n-propyl cation
Answer
B
Question. The correct stability order for the following species as
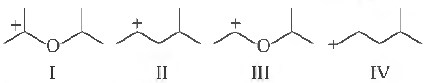
(a) II > IV > I > III
(b) I > II > III > IV
(c) II > I > IV> III
(d) I > III > II > IV
Answer
D
Question. The compound without a chiral carbon atom is
CH3
l
(a) Br CH2 CHCH2Br
CH3
l
(b) C2H5CH2 CHCH2 Br
(c) CH3CH2 CHCH2Br
l
CH3
(d) HOOC CH (Br) COOH
l
CH3
(e) OHC—CH(OH)—CH2OH
Answer
A
Question. How many chiral compounds are possible on monochlorination of 2-methyl butane?
(a) 8
(b) 2
(c) 4
(d) 6
Answer
C
Question. Total number C4H10O is of isomers for the molecular formula
(a) 3
(b) 4
(c) 6
(d)7
Answer
D


