Please see Chapter 5 Periodic Classification of Elements Exam Questions Class 10 Science below. These important questions with solutions have been prepared based on the latest examination guidelines and syllabus issued by CBSE, NCERT, and KVS. We have provided Class 10 Science Questions and answers for all chapters in your NCERT Book for Class 10 Science. These solved problems for Periodic Classification of Elements in Class 10 Science will help you to score more marks in upcoming examinations.
Exam Questions Chapter 5 Periodic Classification of Elements Class 10 Science
ONE MARK QUESTIONS
Question: Write two reasons responsible for the late discovery of noble gases.
Answer: a. They are inert i.e., least reactive.
b. They are less abundant in nature except Argon.
Question: The atomic number of three elements A, B and C are 12, 18 and 20 respectively. State giving reason, which two elements will show similar properties.
Answer: A(12): 2, 8, 2, B(18): 2, 8,8, C(20): 2, 8, 8, 2
A and C belong to the same group because they have same number of valence electrons.
Question: Why are H, Li, Na and K placed in group-1?
Answer: It is because they have one valence electron.
Question: Write the number of horizontal rows in the modern
periodic table. What are these rows called?
Answer: There are 7 horizontal rows in the modern periodic Table. These rows are called periods.
Question: List any two properties of elements belonging to the first group of modem periodic table.
Answer: a. They have 1 valence electron.
b. Their valency is equal to 1.
Question: Write the atomic numbers of two elements X and Y having electronic configurations 2, 8, 2 and 2, 8, 6, respectively.
Answer: X = 2 + 8 + 2 = 12
Y = 2 + 8 + 6 = 16
Question: State the modern periodic law of classification of elements.
Answer: Modern Periodic Law: It states ‘properties of elements are a periodic function of their atomic number’.
Question: Write one difference between Group 1 and Group 2 elements?
Answer: Group 1 elements has 1 valence electron whereas group 2 elements has 2 valence electrons.
Question: Write the formula which is used to determine the maximum number of electrons that a shell can accommodate.
Answer: 2n2 , where n is the shell number.
Question: What would be the nature of oxides formed by the elements on the right side of periodic table?
Answer: On right hand side of the periodic table, most of them are non-metals, therefore they form acidic oxides.
Question: Out of the three elements P, Q and R having atomic numbers 11, 17 and 19 respectively, which two elements will show similar properties and why?
Answer: P(11): 2, 8, 1; Q(17): 2, 8, 7; R(19): 2, 8, 8, 1
P and R will show similar chemical properties because they have the same number of valence electrons.
Question. Write the number of horizontal rows in the modern periodic table. What are these rows called?
Answer: There are 7 horizontal rows in the modern periodic Table. These rows are called periods.
Question. On the basis of electronic configuration, how will you identify the first and the last element of a period?
Answer: 1st element will have 1 valence electron whereas last element will have 8 valence electrons except in first period in which last element has 2 electrons.
Question. Write one difference between Group 1 and Group 2 elements?
Answer: Group 1 elements has 1 valence electron whereas group 2 elements has 2 valence electrons.
Question. State Mendeleev’s periodic law.
Answer: Mendeleev’s Periodic Law: Properties of elements are a periodic function of their atomic mass.
Question. Out of the three elements P, Q and R having atomic numbers 11, 17 and 19 respectively, which two elements will show similar properties and why?
Answer: P(11): 2, 8, 1; Q(17): 2, 8, 7; R(19): 2, 8, 8, 1 P and R will show similar chemical properties because they have the same number of valence electrons.
Question. An element has atomic number 17. To which group and period does it belong to?
Answer: X(17): 2,8,7 is the electronic configuration. It has 7 valence electrons. It belongs to 17th group and 3rd period.
Question. Lithium, Sodium and potassium form a Dobereiner’s triad. The atomic masses of lithium and potassium are 7 and 39 respectively. Predict the atomic mass of sodium.
Answer: Atomic mass of Sodium Atomic mass of lithium
= + Atomic mass of potassium/2
Na = 7+ 39/2 = 46/2 = 23
Question:The electronic configuration of two elements X and Y are 2, 8, 7 and 2, 8, 8, 3 respectively. Write the atomic numbers of X and Y.
Answer: X = 2 + 8 + 7 = 17
Y = 2 + 8 + 8 + 3 = 21
TWO MARKS QUESTIONS
Question: Why do all elements of the
a. same group have similar properties?
b. same period have different properties?
Answer: a. It is due to same number of valence electrons which will decide the chemical properties.
b. They differ in number of valence electrons, therefore they differ in chemical properties. They have the same number of shells.
Question: How can the valency of an element be determined if its electronic configuration is known? What will be valency of an element with atomic number 9?
Answer: Valency=Number of valence electrons in case of metals and metalloids. It is also equal to 8 — Number of valence electrons in case of non-metals.
F(9) has electronic configuration of 2, 7. It is a non metal.
Its valency is equal to 1.
Question: An element ‘E’ has the following electronic configuration:

a. To which group of the periodic table does element E belong to?
b. To which period of the periodic table does element E belong to?
c. State the number of valence electrons present in element E.
d. State the valency of the element E.
Answer: a. E (2, 8, 6) belongs to group 16,
b. It belongs to 3rd period.
c. It has 6 valence electrons.
d. The valency of E is 2.
Question. Give reason why noble gases are placed in a separate group in the modern periodic table.
Answer: It is because they have their outermost shell completely filled and resemble with each other.
Question. Name any two pairs of elements which were adjusted by Newlands in the same slot.
Answer: (i) Co and Ni, (ii) Ce and La
Question. Why was the system of classification of elements into triads not found suitable?
Answer: It is because all the elements discovered at that time could not be classified as Dobereneire’s triads.
Question. Out of Li and K, which will have stronger metallic character and why?
Answer: K will have more metallic character because it can lose electrons easily due to its bigger atomic size and less effective nuclear charge.
Question. Why does atomic size decreases as we move from left to right along a period in a periodic table?
Answer: It is because one proton and one electron is being added successively, therefore effective nuclear charge increases, atomic size decreases.
Question. What were the criteria used by Mendeleev in creating his periodic table?
Answer: Increasing order of atomic mass and same formula of oxides and hydrides.
Question. Write two reasons responsible for the late discovery of noble gases.
Answer:
a. They are inert i.e., least reactive.
b. They are less abundant in nature except Argon.
Question: An element has atomic number 13.
a. What is the group and period number to which this element belongs to?
b. Is the element a metal or a non-metal?
Answer: a. It belongs to group 13, 3rd period because it has 3 valence electrons and 3 shells.
b. It is a metal because it can lose 3 electrons to become stable.
Question: The electronic configuration of two elements A and B are 2, 8, 7 and 2, 8, 8, 2 respectively. Write the atomic number of these elements. What will be formula of the compound formed and the nature of bond between them when these two elements chemically combine together?
Answer: A – 2 + 8 + 7 = 17 is its atomic number.
B – 2 + 8 + 8 + 2 = 20 is its atomic number.
A(2, 8, 7) B(2, 8, 8, 2)
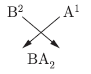
The nature of bond is ionic bond.
Question: The atomic number of three elements are given below:
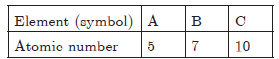
Write the symbol of element which belongs to (a) group 13, (b) group 15 of the periodic table. State the period to which these elements belongs to. Give reason for your answer.
Answer: a. A(2, 3) belongs to group 13 because it has 3 valence electrons.
b. B(2, 5) belongs to group 15 because it has 5 valence electrons. It belongs to 2nd period because it has 2 shells.
Question: How the electronic configuration of the atom of an element is related to its position in the modern periodic table? Explain with one example.
Answer: Period No. = Number of shells
Group No. = Number of valence electrons or valence electrons +10.
Example
Na(11): 2, 8,1. It has 1 valence electron, it belongs to group 1. Also Al (13): 2, 8, 3. It has 3 shells, therefore it belongs to 3rd period.
Question: On the basis of electronic configuration, how will you identify the first and the last element of a period?
Answer: 1st element will have 1 valence electron whereas last element will have 8 valence electrons except in first period in which last element has 2 electrons.
Question: In the modern periodic table, the element Calcium (atomic number = 20) is surrounded by elements with atomic numbers 12, 19, 21 and 38. Which of these elements has physical and chemical properties resembling those of Calcium and why?
Answer: Ca(20): 2, 8, 8, 2
Mg(12): 2,8, 2
Sr(38): 2, 8, 18, 8, 2
Mg and Sr has similar properties to Ca because each of them have 2 valence electrons.
Question: The atomic number of these elements are given below:
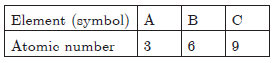
Write the symbol of element which belongs to
a. group 1,
b. group 14 of the periodic table.
State the period of the periodic table to which these elements belong to. State reason to support your answer.
Answer: c. A(3) has electronic configuration 2, 1. It belongs to group 1. Hence the number of valence electrons is 1.
d. B(6) has electronic configuration 2, 4. It belongs to group 14, valence electrons = 4.
They belong to second period because they have 2 shells.
Question: How does metallic character of the elements change along a period in the periodic table from left to right and why?
Answer: Metallic character of elements decreases along a period from left to right because atomic size decreases, tendency to lose electrons decreases.
Question: How does valency of the elements vary a. in going down a group, and b. in going from left to right in a period of the periodic table?
Answer: a. It remains the same.
b. It first increases till middle, then decreases.
Question: The atomic number of three elements, X, Y and Z are 9, 11 and 17 respectively. Which two of these elements will show similar chemical properties? Why?
Answer: X(9) : 2, 7
Y(11) : 2, 8, 1
Z(17) : 2, 8, 7
X and Z have similar chemical properties because theyhave the same number of valence electrons.
Question: In the periodic table, how does the tendency of atoms to lose electrons change on going from
a. left to right across a period?
b. top to bottom in a group?
Answer: a. Tendency to lose electron(s) decreases across the period from left to right.
b. Tendency to lose electron(s) increases down in a group.
Question: Give reasons:
a. Elements in a group have similar chemical properties.
b. Elements of Group 1 form ions with a charge of +1.
Answer: a. It is due to the same number of valence electrons.
b. Group-1 elements can lose one electron to form positive ions with charge equal to +1.
Question: What is meant by periodicity of properties of elements? Why are the properties of elements placed
on the same group of the periodic table similar?
Answer: The repetition of similar properties after a definite interval is called periodicity of properties. It is due to the same number of valence electrons.
Question: The elements of the second period of the periodic table are given below:
Li, Be, B, C, N, O, F
a. Give reason to explain why atomic radii decreasesfrom Li to F.
b. Identify the most (i) metallic and (ii) non-metallic element.
Answer: a. It is because effective nuclear increases due to increase in forces of attraction between more electrons with more protons, even though number of shells remain the same.
b. (i) Li is the most metallic element, (ii) F is the most non-metallic element.
Question: Elements Mg and O respectively belong to group 2 and group 16 of the modem periodic table. If the atomic number of Mg and O are 12 and 8 respectively, draw their electronic structures and show the process of formation of the compound by transfer of electrons between them.
Answer: Mg(12): 2,8,2
O(8): 2,6
Question:State Mendeleev’s periodic law. Write two achievements of Mendeleev’s periodic table.
Answer: Properties of elements are a periodic function of their atomic mass.
Achievements:
a. It could arrange all the elements discovered at that time.
b. It left some gaps for the elements to be discovered and helped in their discovery by predicting their properties.
Question: Given below are four elements with their atomic numbers:
A(16), B(11), C(3), D(14)
a. Identify the elements which belong to the same
group of the Modem periodic table.
b. Arrange the elements in decreasing order of the atomic size.
c. Write the formula of oxide of B.
d. Which of these elements is a metalloid?
Answer: a. C(3) and B( 11) belongs to the same group.
b. A<D<B<C is the decreasing order of atomic size.
c. B2O is the formula of oxide.
d. D is a metalloid.
Question: Give reasons for the following:
a. Lithium atom is smaller than sodium atom.
b. Chlorine (Atomic number 17) is more
electronegative than Sulphur (Atomic number 16)
Answer: a. Li(3): 2, 1; Na(11): (2, 8, 1).
Li has two shells, therefore it is smaller than Na which has 3 shells.
b. Chlorine (17) is smaller than sulphur (16), therefore it is more electronegative than sulphur.
Question: Would you place the two isotopes Cl-35 and Cl-37 in different slots because of their different atomic masses or in the same slot because their chemical properties are same? Justify your answer.
Answer: They will be placed in the same slot because they have similar chemical properties due to same number of valence electrons.
Question: The elements of the third period of the Periodic Table are given below:
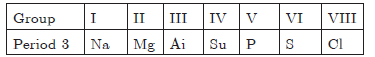
a. Which atom is bigger, Na or Mg? Why?
b. Identify the most (i) metallic and (ii) non-metallic element in Period 3.
Answer: a. Na is bigger because it has 11p and 11e i.e., less forces of attraction than in Mg which has 12 protons and 12 electrons and has more forces of attraction, due to more effective nuclear charge.
b. (i) Na is the most metallic element, (ii) Cl is the most non-metallic element.
Question: Two elements M and N belong to group 1 and 2 respectively and are in the same period of the periodic table. How do the following properties of M and N vary?
a. Sizes of their atoms.
b. Their metallic character.
c. Their valencies in forming oxides.
d. Formulae of their chlorides.
Answer: a. M has bigger size than N.
b. M has more metallic character than N.
c. M has valency equal to 1, N has valency equal to 2.
d. MCl and MCl2 are formula of their chlorides.
Question: a. State the main characteristic of elements on which modern periodic table is based.
b. No fixed position is assigned to hydrogen in the periodic table, why?
Answer: a. Modem periodic table is based on the trend of increasing order of atomic number. Elements of same group have same number of valence electrons.
b. Hydrogen resembles with both group 1 and group 17 elements, therefore it does not have a fixed position.
THREE MARKS QUESTIONS
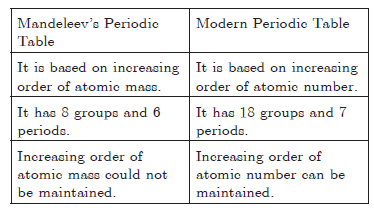
Question: Differentiate between the arrangement of elements in Mendeleev’s periodic table and Modern Periodic Table.
Answer:
Question: The atomic number of an element is 12.
a. Write its electronic configuration and determine its valency.
b. Is it more reactive or less reactive than Ca(20)?
c. Is it a metal or a non-metal?
d. Write the formula of its oxide.
Answer: a. Mg(12): 2, 8, 2. Its valency is equal to 2.
b. It is less reactive than Ca.
c. It is a metal.
d. MgO
Question: An element ‘P’ (atomic number 20) reacts with an element ‘Q’ (atomic number 17) to form a compound.
Answer the following questions giving reason:Write the position of ‘P’ and ‘Q’ in the Modem Periodic Table and the molecular formula of the compound formed when ‘P* reacts with ‘Q’.
Answer: P(20): 2, 9, 9, 2; Q(17): 2, 8, 7
‘P’ belongs to group 2 and 4th period.
‘Q’ belongs to group 17 and 3rd period.
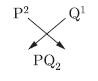
PQ2 is the molecular formula of the compound formed.
Question: Write the electronic configuration of two elements X’ and ‘Y’ whose atomic numbers are 20 and 17 respectively. Write the molecular formula of the compound formed when element X’ reacts with element ‘Y’. Draw electron-dot structure of the product and also state the nature of the bond formed between both the elements.
Answer:
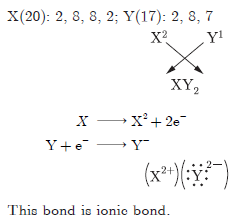
Question: Write the names given to the vertical columns and horizontal rows in the Modern Periodic Table. How does the metallic character of elements vary on moving down a vertical column? How does the size of atomic radius vary on moving from left to right in a horizontal row? Give reason in support of your answer in the above two cases.
Answer: Vertical columns are called groups. Horizontal rows are called periods. Metallic character of elements increases down the group because tendency to lose electrons increases down the group due to increase in atomic size. Atomic size goes on decreasing along the period due to increase in effective nuclear charge due to increase in number of protons and electrons.
Question: An element X belongs to 3rd period and group 16 of the Modern Periodic table.
a. Determine the number of valence electrons and the valency of X.
b. Molecular formula of the compound of X when it reacts with hydrogen and write its electron dot diagram.
c. Name the element X and state whether it is metallic or non-metallic.
Answer: a. X has 6 valence electrons and its valency is equal to 2.
b. H2X is its formula,
c. X is sulphur. It is a non-metallic element.
Question: Why is atomic number considered to be a more appropriate parameter than atomic mass for the classification of elements in a periodic table? How does the metallic character of elements vary as we move (a) from left to right in a period, and (b) top to bottom in a group of the modem periodic table? Give reasons to justify your answer.
Answer: It is because chemical properties depend upon the number of valence electrons which is determined with the help of atomic number.
a. Metallic character decreases from left to right because atomic size decreases, tendency to lose electrons decreases,
b. Metallic character increases from top to bottom in a group because atomic size increases due to which effective nuclear charge decreases.
Question: Two elements X and Y have atomic number 11 and 16 respectively.
a. Write the electronic configuration of both.
b. Write the formula of the compound formed by their combination (in terms of X and Y).
Answer: a. X (11): 2, 8, 1; Y (16): 2, 8, 6
b. X2Y is the formula of compound formed.
Question: The atomic number of Na and Mg are 11 and 12 respectively and they belong to the same period
a. Which one should have smaller atomic size?
b. Which would be more electropositive?
c. To which group would each one belong?
Answer: Na(11): 2, 8, 1; Mg(12): 2, 8, 2
a. Mg will have smaller size.
b. Na is more electropositive.
c. Na(11) belongs to group 1 whereas Mg(12) belongs to group 2.
Question:Describe the basic character of oxides of third period elements across the period from left to right.
Answer: Na2O, MgO are basic oxides.
SiO2, Al2O3 are amphoteric oxides.
P2O5, SO2, Cl2O7 are acidic oxides.
Basic character of oxides decreases across the period.
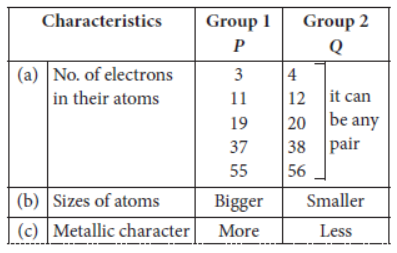
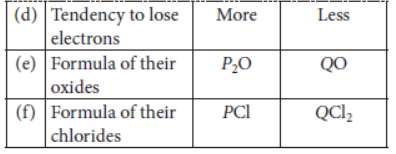
Question: Two elements P and Q belong to the same period of the modern periodic table and are in Group-1
and Group-2 respectively. Compare their following characteristics in tabular form:
a. The number of electrons in their atoms.
b. The size of their atoms.
c. Their metallic character.
d. Their tendency to lose electrons.
e. The formula of their oxides.
f. The formula of their chlorides.
Answer: a. P has 1 valence electron, Q has 2 valence electrons.
b. P is bigger than Q.
c. P is more metallic than Q.
d. P can lose electrons more easily than Q.
e. P2O and QO
f. PCl and QCl2
Question: a. How many periods are there in the Modem Periodic Table of elements?
b. How do atomic radius, valency and metallic character vary down a group?
c. How do atomic size and metallic character of elements vary as we move from left to right in a period?
Answer: a. There are 7 periods.
b. Atomic radius and Metallic character increases down the group. Valency remains the same in a group.
c. Atomic size and metallic character decrease along a period from left to right in a period.
Question: Na/Mg and A1 are the elements having one, two and three valence electrons respectively. Which of these elements
(a) has the largest atomic radius,
(b) is least
reactive? Justify you answer stating reason for each.
Answer: Na has largest atomic radii due to 11 protons and 11 electrons and least effective nuclear charge. Na is most reactive because it can lose an electron easily due to its large size and least effective nuclear charge.
Question: a. How are the following related?
i. Number of valence electrons of different elements in the same group.
ii. Number of shells of elements in the same period.
b. How do the following change?
i. Number of shells of elements as we go down a group.
ii. Number of valence electrons of elements on moving from left to right in a period.
iii. Atomic radius in moving from left to right along a period.
iv. Atomic size as we go down a group.
Answer: a. i. Same number of valence electrons.
ii. Number of shells remains the same in a period.
b. i. Number of shells goes on increasing down the group.
ii. Number of valence electrons goes on increasing in a period from left to right.
iii. Atomic radius decreases along a period from left to right.
iv. Atomic size increases down a group.
Question: Explain the variation in the following properties of the elements in periodic table:
a. Atomic radius in the periodic table,
b. Metallic character in a period,
c. Valency in a group.
Answer: a. Atomic radius and Metallic character increases down the group. Valency remains the same in a group.
b. Atomic size and metallic character decrease along a period from left to right in a period.
Question: The elements of group 18 of the periodic table are given:
He, Ne, Ar, Kr, Xe, Rd, Dg
a. The elements of this group are unreactive, why?
b. Which atom is bigger in size Ne or Ar and why?
Answer: a. It is because they have their octet complete i.e., stable electronic configuration.
b. Ar has bigger atomic size than Ne because Ar has 3 shells while Ne has 2 shells; Ar (2, 8, 8), Ne (2, 8)
Question: a. How does electropositivity of elements gets affected as we move (i) down the group, (ii) across the period?
b. Which atomic property increases both ways: as we move across the period or down the group?
Answer: a. (i) Electropositivity increases down the group.
(ii) Electropositivity decreases across the period from left to right.
b. Atomic number increases across the period as well as down the group.
Question: (a) State modern periodic law.
(b) How many groups and periods are present in the modern periodic table?
(c) State how the problem of placing (a) hydrogen,
(b) isotopes of an element has been solved in this periodic table.
Answer: a. Properties of elements are a periodic function of their atomic numbers’.
b. There are 18 groups and 7 periods.
c. (i) Hydrogen is placed along with group 1 as well as halogens because it resembles with both of them.
(ii) Isotopes do not need a separate place as they have the same atomic number.
Question: a. Predict which of the following will form anions and which will form cations.
(i) Na, (ii) Al, (iii) Cl, (iv) O
b Name two elements that are inert.
Answer: a. Cl and O will form anions i.e., Cl– and O2- Na and A1 will form cations i.e., Na+ and Al3 +.
b. He and Ne are inert elements.
Question: (a) Identify the element that have two completely filled shells and the number of valence electrons in each case if atomic numbers are:
(i) 1, (ii) 2,
(iii) 7, (iv) 8
(b) Analyse which amongst them is inert.
Answer: a. (i) 2, 8, 1: Sodium
(ii) 2, 8, 2: Magnesium
(iii) 2, 8, 7: Chlorine
(iv) 2, 8, 8: Argon
(b) Argon is inert.
Question: The atomic number of K and Ca is 19 and 20 respectively and they belong to same period.
a. Which amongst them would have smaller atomic size?
b. Which one would be more electro positive?
c. To which group would each one belong to?
Answer: a. Ca has smaller atomic size.
b. K is more electropositive.
c. K belongs to group 1, Ca belongs to group 2.
Question: a. Why did Mendeleev leave gaps in his periodic table?
b. State any three limitations of Mendeleev’s periodic table.
c. How do the electronic configuration of atoms
change in a period with increase in atomic number.
Answer: a. He left gaps for the new elements which are to be discovered.
b. (i) Increasing order of atomic mass could not be maintained.
(ii) Position of hydrogen was not justified.
(iii) Isotopes could not be placed in different slots due to different atomic mass but same properties.
c. Number of valence electrons keep on increasing along a period from left to right in a period.
Question: The position of three elements A, B and C in the periodic table are indicated below:
Answer:
Group 16 Group 17
– – (First Period)
– A (Second Period)
– – (Third Period)
B C Fourth Period)
a. State whether element C would be a metal or a non-metal. Why?
b. Which is the more active element, A or C? Why?
c. Which type of ion (cation or anion) will be formed by the element C? Why?
Answer: a. O is a non-metal because it has 7 valence electrons.
It can gain one electron to form an anion.
b. A is more reactive than C because it is smaller in size, therefore it can gain electron(s) easily.
Question: Atoms of seven elements A, B, C, D, E, F and G have a different number of electronic shells but have the same number of electrons in their outermost shells.
The elements A and C combines with chlorine to form an acid and common salt respectively. The oxide of element A is liquid at room temperature and it is a neutral substance, while the oxides of the remaining six elements are basic in nature. Based on the above information, answer the following question:
a. What could the element A be?
b. Will elements A to G belong to the same period or same group of the periodic table?
c. Write the formula of the compound formed by the reaction of the element A with oxygen.
d. Show the formation of the compound by the combination of element C with chlorine with the help of electronic structure.
e. What would be the ratio of number of combining atoms in a compound formed by the combination of element A with carbon?
f. Which one of the given elements is likely to have the smallest atomic radius?
Answer: a. A is hydrogen.
b. A and G will belong to the same group.
c. H2O is the formula of the compound.
d. C is sodium.
Na → Na+ + e– or C → C+ + e-
Cl + e–→ Cl– or Cl +e– → Cl–.

e.CA4 i.e., 1:4
f. A has smallest atomic radius.
Question: In the following table, six elements A, B, C, D, E and F (here letters are not the usual symbols of the elements) of the Modern Periodic Table with atomic numbers 3 to 18 are given as follows:

a. Which of these is (i) a noble gas, (ii) a halogen?
b. If B combines with F, what would be the formula of the compound formed?
c. Write the electronic configurations of ‘C’and’E’.
Answer: a. (i) G is a noble gas, (ii) F is a halogen.
b. BF is the formula of compound.
c. C: 2, 8, 2 E: 2, 8, 6
Question: In the following table, are given eight elements A, B, C, D, E, F, G and H (here letters are not the usual symbols of the elements) of the Modem Periodic Table with the atomic numbers of the elements in parenthesis.
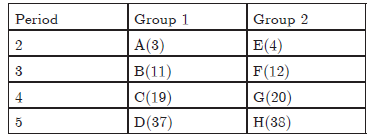
a. What is the electronic configuration of‘F?
b. What is number of valence electrons in ‘F’?
c. What is number of shells in *F’?
d. Arrange E, F, G, H in decreasing order of atomic size.
e. State whether F is a metal or a non¬metal.
f. Out of three elements B, E and F, which one has biggest atomic size.
Answer: a. 2, 8, 2 is the electronic configuration of F.
b. No. of valence electrons = 2.
c. Three shells are present in F.
d. H > G > F > E is the decreasing order of atomic size.
e. F is a metal.
f. B has biggest atomic size.
Question: a. What is meant by periodicity in properties of elements with reference to the periodic table?
b. Why do all the elements of the same group have similar properties?
c. How will the tendency to gain electrons change as we go from left to right across a period. Why?
Answer: a. The repetition of similar properties after regular intervals is called periodicity in properties.
b. It is because they have the same number of valence electrons.
c. Tendency to gain electrons increases from left to right across the period due to decrease in atomic size.
Question: The position of three elements A, B and C in the Periodic Table is shown below:

Giving reasons, explain the following:
a. Element ‘A’ is a non-metal.
b. Element ‘B’ has a larger atomic size than element ‘C’.
c. Element ‘C’ has a valency of 1.
Answer: a. A is a non-metal because it has 7 valence electrons, it can gain one electron to form anion.
b. B has less electrons and protons, less forces of attraction between the nucleus and valence electrons, therefore it is bigger in size.
c. C can gain 1 electron to become stable, therefore its valency is equal to 1.
Question: Table given below shows a part of the modern periodic table.
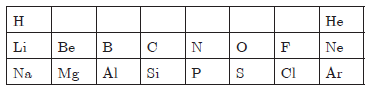
Using this table, explain why
a. Li and Na are considered as active metals.
b. Atomic size of Mg is less than that of Na.
c. Fluorine is more reactive than chlorine.
Answer: a. Li and Na have largest atomic size in respective period, therefore they can lose an electron easily, hence they are active metals.
b. Mg has 12 protons and 12 electrons which has more forces of attraction, therefore, it is smaller in size than Na which is having 11 protons and 11 electrons.
c. F is smaller in size, it can gain electrons easily, therefore, it is more reactive than Cl.
Question: The position of three elements A, B and C in the Periodic Table is shown below:
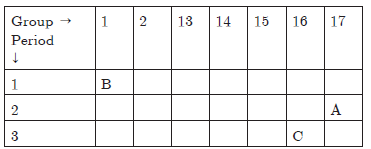
Giving reasons, explain the following:
a. Element A is a non-metal.
b. Atoms of element C has a larger size than atoms of element A.
c. Element B has a valency of 1.
Answer: a. A is a non-metal because it can gain one electrons to form anion.
b. C has more number of shells i.e., it has 3 shells because it belongs to 3rd period whereas A has 2 shells as it belongs to 2nd period.
c. B can lose 1 electron to become stable, therefore its valency is equal to 1.
Question: What physical and chemical properties of elements were used by Mendeleev in creating his periodic table? List two observations which posed a challenge to Mendeleev’s Periodic Law.
Answer: a. Increasing order of atomic mass was the physical property.
b. Formulae of oxides and hydrides was the chemical property.
i. Increasing order of atomic mass could not be maintained.
ii. Position of isotopes posed a challenge for the classification of elements.
Question: Examine elements of the third period: Na, Mg, Al, Si,
P, S, Cl and Ar Answer the following:
a. Choose (i) Metals, (ii) Non-metals out of these elements.
b. On which side of periodic table we find (i) metals (ii) non-metals.
c. Name metalloids out of the elements given above.
Where are they located in the periodic table?
Answer: a. (i) Na, Mg, A1 are metals.
(ii) P, S, Cl, Ar are non-metals.
b. (i) Metals are placed on the left hand side and middle part of the periodic table.
(ii) Non-metals are placed on the right hand side of periodic table.
c. Silicon is a metalloid. They are located between metals and non-metals at the border line in a zigzag manner.
Question: The element Be, Mg and Ca are placed in the second group of the periodic table. Their atomic numbers are 4, 12, 20 respectively a. Write the electronic configuration of these elements.
b. Write the valency exhibited by them,
c. Which three elements will be the most reactive?
Answer: a.
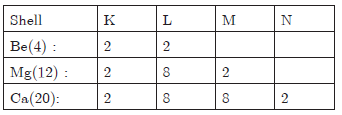
b. Valency is 2.
c. Ca is the most reactive metal.
Question: In the figure given below the first 20 elements are jumbled up. Carefully observe the figure and answer the following questions:
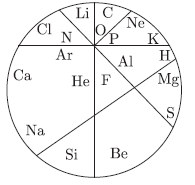
a. Pick out the two elements which are known as alkali metals.
b. Pick out the two elements which have valency of 4.
c. Pick out two elements which belong to group 16 of the periodic table.
Answer: a. Na, K are alkali metals.
b. C, Si have valency equal to 4
c. O and S belongs to group 16.
Question: Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electronic shells but different number of electrons in their outermost shell. It was
, found that elements A and G combine to form an ionic compound which can also be extracted from sea water. Oxides of the elements A and B are basic in nature while those of E and F are acidic. The oxide of element D is almost neutral. Answer the following questions based on the information given herein:
a. To which group or period of the periodic table do the listed elements belong?
b. Which one of the eight elements is likely to be a noble gas?
c. Which of the eight elements would have the largest atomic radius?
d. Which two elements amongst these are likely to be non-metals?
e. Which one of these eight elements is likely to be a semi-metal or a metalloid?
Answer: a. A and B belongs to group-1 and. group-2 respectively because they form basic oxides. ‘C’ belongs to group-13, ‘D’ belongs to group-14 which forms almost neutral oxide (actually amphoteric oxide), E and F belong to group-15, 16 forming acidic oxides. ‘G’ belongs to group-17 because NaCl is used in cooking. ‘H’ belongs to group 18.
they belong to third period of the periodic table.
b. H belongs to noble gas elements.
c. A will have largest atomic radius.
d. E and F are likely to be non-metals,
e. D is likely to be a metalloid or semi metal.
Question: As we move across a period in the periodic table, what is gradation in the following properties:
(a) Atomic
size, (b) Atomic number, (c) Electronegativity?
Answer: a. Atomic size goes on decreasing across the period from left to right.
b. Atomic number goes on increasing along the period from left to right.
c. Electronegativity goes on increasing along a period from left to right.
Question: The position of elements A, B, C, D, E, F and G in the Modern Periodic Table is given as under:

a. In which group are inert elements placed?
b. What type of ions would ‘B’, ‘C’, ‘E’ and ‘F’will form?
c. Which element would have chemical properties similar to ‘C’?
d. How many shells do ‘A’ have?
e. What is the similarity between ‘A’ and ‘D’?
f. Identify the most abundant element in the earth crust.
Answer: a. Group 18
b. They will form anions.
c. F will have similar properties to ‘C’.
d. A has only one shell.
e. A and D both are inert elements.
f. C is the most abundant element in the Earth crust.
Question: (a) Name metals among the first five elements of the Modern Periodic Table.
(b) Write their symbols.
(c) Write the formula of their oxides.
Answer: a. Lithium and Beryllium are metals among first five elements.
b. Lithium (Li), Beryllium (Be).
c. Li2O and BeO are the formulae of their oxides.
Question: Lithium, Sodium, Potassium are placed in the same group on the basis of their similar properties. List three such similar properties.
Answer: a. All of them are reactive metals.
b. They have 1 valence electron and form positive ions with +1 charge.
c. They are largest in size in their respective periods.
FIVE MARKS QUESTIONS
Question. How does the tendency of the elements to loose electrons change in the Modern Periodic Table in (i) a group, (ii) a period and why?
Answer: (i) Tendency of the elements to loose electrons increases down the group. The reason being that at each succeeding element down a group, the number of shells increases. So, the distance of the valence shell from the nucleus increases due to which the effective nuclear charge decreases on the last shell of electrons. So, it becomes easier for the atom to loose electrons.
(ii) Tendency of the elements to loose electrons decreases in a period from left to right. The reason being that as the electron enters to the same shell at each successive element so, the effective nuclear charge on the valence shell electron increases, the attraction between the valence electrons and nucleus increases so, it becomes dificult to loose electrons.
Question. How many groups and periods are there in the Modern Periodic Table? How do the atomic size and metallic character of elements vary as we move :
(a) down a group and
(b) from left to right in a period?
Answer: There are 18 groups and 7 periods in the Modern periodic table.
– Atomic size increases down the group, while moving from left to right in a period it decreases.
– Metallic character of elements increases down the group while moving from left to right in a period it decreases.
Question. Write the number of periods the Modern Periodic Table has. State the changes in valency and metallic character of elements as we move from left to right in a period. Also state the changes, if any, in the valency and atomic size of elements as we move down a group.
Answer: There are 7 periods in the Modern periodic table.
As we move along the period from left to right then valency of the elements ¬rst increases from 1 to 4 and then decreases to 0.
On moving from left to right in a period the metallic character of elements decreases as the electropositive character of elements decreases across the period.
On moving down the group, the valency of the elements remains the same while atomic size increases. This is due to addition of new shell of electrons at every successive step.
Question. Two elements ‘P’ and ‘Q’ belong to the same period of the Modern Periodic Table and are in Group-1 and Group-2 respectively.
Compare their following characteristics in tabular form :
(a) The number of electrons in their atoms.
(b) The sizes of their atoms.
(c) Their metallic character.
(d) Their tendencies to loose electrons.
(e) The formula of their oxides.
(f) The formula of their chlorides.
Answer: The given characteristics can be tabulated as follows :
Question. Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position in the Modern Periodic Table and how valency of an element is calculated on the basis of its atomic number.
Answer: Atomic number of the element = 16
Thus, electronic configuration = 2, 8, 6
Since, this element contains 3 shells hence, it belongs to period number 3.
As the element has 6 valence electrons, group number = 10 + 6 = 16
The valency of an element is determined by the number of electrons present in the outermost shell.
Th Valency of the element = 8 – valence electrons = 8 – 6 = 2
Question. Given below are some elements of the Modern Periodic Table. Atomic number of the element is given in the parentheses :
A(4), B(9), C(14), D(19), E(20)
(a) Select the element that has one electron in the outermost shell. Also write the electronic configuration of this element.
(b) Which two elements amongst these belong to the same group? Give reason for your answer.
(c) Which two elements amongst these belong to the same period? Which one of the two has bigger atomic radius?
Answer: The electronic configuration of the given elements will be as follows :
A(4) = 2, 2
B(9) = 2, 7
C(14) = 2, 8, 4
D(19) = 2, 8, 8, 1
E(20) = 2, 8, 8, 2
(a) Element D will have one electron in its outermost shell.
(b) Elements A and E will belong to same group as both of them have same electrons in their outermost shells.
(c) A and B belong to period number 2 (two shells).
D and E belong to period number 4 (four shells).
Question. An element ‘X’ belongs to third period and second group of the Modern Periodic Table.
(a) Write its electronic configuration.
(b) Is it a metal or non-metal? Why?
(c) Write the formula of the compound formed when ‘X’ reacts with an element (i) Y of electronic configuration 2, 6 and (ii) Z of electronic configuration 2, 8, 7.
Answer: Third period indicates that it has three shells while group 2 indicates that it has two valence electrons in its outermost shell.
Thus, X must be magnesium (Mg).
(a) Electronic configuration = 2, 8, 2
(b) As X has two valence electrons in its outermost shell which can be easily lost to form a noble gas configuration, so it will be a metal.
(c) (i) Electronic configuration of Y = 2, 6
Hence, valency of Y = 8 – 6 = 2
Formula of compound formed when X reacts with Y is
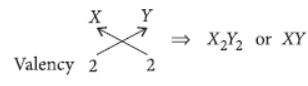
(ii) Electronic configuration of Z = 2, 8, 7
Hence, valency of Z = 8 – 7 = 1
Formula of compound formed when X reacts with Z is
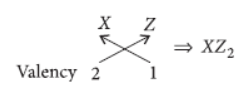
Question. In the following table, the positions of six elements A, B, C, D, E and F are given as they are in the Modern Periodic Table :
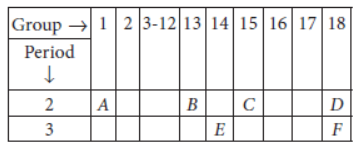
following questions :
(i) Name the element which forms only covalent compounds.
(ii) Name the element which is a metal with valency three.
(iii) Name the element which is a non-metal with valency three.
(iv) Out of B and C, whose atomic radius is bigger and why?
(v) Write the common name for the family to which the elements D and F belong.
Answer: (i) Element E will form only covalent compounds because it has 4 electrons in the outermost shell so, it can neither loose nor gain 4 electrons, hence E forms compounds by sharing of electrons.
(ii) Element B is a metal having valency 3 as it belongs to group 13.
(iii) C is a non-metal with valency (8 – 5 =) 3.
(iv) Out of B and C, B will be bigger in size because as we move along the period from left to right, the atomic radius decreases due to addition of electrons in the same shell at each successive element. Hence, nucleus pulls electrons more towards the centre.
(v) D and F belong to group 18 and are called noble gases.
Question. Na, Mg and Al are the elements of the same period of Modern Periodic Table having one, two and three valence electrons respectively.
Which of these elements (i) has the largest atomic radius, (ii) is least reactive? Justify your answer stating reason for each case.
Answer: Na, Mg and Al belong to same period of Modern periodic table.
Valence electrons Na Mg Al
1 2 3
(i) Sodium (Na) will have the largest atomic radius because as we move from left to right in a period, atomic size decreases due to increase in effective nuclear charge which pulls the outermost electrons more closer to the nucleus.
(ii) Aluminium (Al) is least reactive because on moving from left to right in the periodic table the nuclear charge increases, so the valence electrons are pulled more closer to the nucleus. Therefore, the tendency to loose electrons decreases and hence, reactivity decreases.
Question. (a) Define the following terms :
(i) Valency; (ii) Atomic size
(b) How do the valency and the atomic size of the elements vary while going from left to right along a period in the Modern Periodic Table?
Answer: (a) (i) Valency : It is defined as the combining capacity of the element which is determined by the number of valence electrons present in the outermost shell of its atom.
(ii) Atomic size : It is defined as the distance between the centre of the nucleus and the outermost shell of an isolated atom.
(b) On moving from left to right in the period, the valency of elements increases from 1 to 4 and then decreases to 0.
This is because the elements in a period do not have the same number of valence electrons hence, they do not show same valency.
The atomic size decreases on moving from left to right along a period due to increase in nuclear charge which tends to pull the electrons closer to the nucleus and reduces the size of the atom.
Question. An element ‘X’ is placed in the 3rd group and 3rd period of the Modern Periodic Table.
Answer the following questions stating reason for your answer in each case :
(a) Write the electronic configuration of the element ‘X’.
(b) Write the formula of the compound formed when the element ‘X’ reacts with another element ‘Y’ of atomic number 17.
(c) Will the oxide of this element be acidic or basic ?
Answer: X is placed in 3rd group (IIIA) and 3rd period of the Modern periodic table then it must be aluminium (Al).
As it belongs to 3rd group so it will have 3 electrons in its outermost shell.
Also it belongs to 3rd period, so it will have 3 shells.
(a) Electronic configuration of X = 2, 8, 3
(b) Atomic number of Y = 17
Electronic configuration = 2, 8, 7
Valency of Y = 8 – 7 = 1
∴ Formula of compound formed when X reacts with Y :

(c) Al2O3 is amphoteric in nature i.e., acidic as well as basic oxide.
Question. Consider two elements X (atomic number 17) and Y (atomic number 20).
(i) Write the positions of these elements in the Modern Periodic Table giving justification.
(ii) Write the formula of the compound formed by the combination of X and Y.
(iii) Draw the electron-dot structure of the compound formed and state the nature of the bond formed between the two elements.
Answer: Atomic number of X = 17
∴ Electronic configuration of X = 2, 8, 7 Atomic number of Y = 20
∴ Electronic configuration of Y = 2, 8, 8, 2
(i) From the electronic configurations, we can easily observe that X contains 3 shells so, it belongs to period 3 and it contains 7 electrons in the outermost shell so, it belongs to group-17. Similarly for Y, it has 4 shells which implies that it belongs to period 4 and Y contains two electrons in the outermost shell so, it belongs to group-2.
(ii) Valency of X = 1
Valency of Y = 2
Thus, formula of the compound formed will be

(iii) Electron dot structure of the compound will be
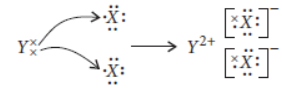
As two electrons present in the outermost shell of Y are donated to two different atoms of X thus, it will be an ionic bond (formed by the complete transfer of electrons).
Question. Consider two elements ‘A’ (Atomic number 17) and ‘B’ (Atomic number 19).
(i) Write the positions of these elements in the Modern Periodic Table giving justification.
(ii) Write the formula of the compound formed when ‘A’ combines with ‘B’.
(iii) Draw the electron dot structure of the compound and state the nature of the bond formed between the two elements.
Answer: Atomic number of A = 17
Electronic configuration of A = 2, 8, 7
Atomic number of B = 19
Electronic configuration of B = 2, 8, 8, 1
(i) From the electronic configuration of A, it can be easily observed that A contains three shells which indicates that it belongs to period 3.
Moreover, it has seven valence electrons in its outermost shell which indicates that it belongs to group 17.
Similarly for B, it has 4 shells so, it belongs to period 4 and it has one electron in outermost shell so, it belongs to group 1.
(ii) The molecular formula of the compound when A combines with B will be

As A contains 7 electrons in the outermost shell so, it is an electronegative element that is why A is placed after B.
(iii) The electron dot structure will be
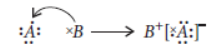
The one electron present in the outermost shell of B gets transferred to the outermost shell of A and hence, ionic bond is formed.
Question. From the following elements :
4Be; 9F; 19K; 20Ca
(i) Select the element having one electron in the outermost shell.
(ii) Two elements of the same group.
Write the formula and mention the nature of the compound formed by the union of 19K and element X (2, 8, 7).
Answer: Thus, formula of compound when K combines with X is

As K has one electron in its outermost shell, so it transfers this electron to outermost shell of X and hence, an ionic compound is formed.
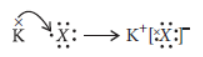
Question. Based on the group valency of elements state the formula for the following giving justification for each :
(i) Oxides of 1st group elements,
(ii) Halides of the elements of group-13, and
(iii) Compounds formed when an element of group-2 combines with an element of group-16.
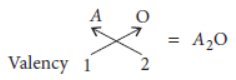
Answer: (i) Oxides of group 1 elements :
Let the element be A.
As A belongs to group 1 of the periodic table, it will have valency = 1.
So, chemical formula of its oxide will be
(ii) Halides of the element of group-13 :
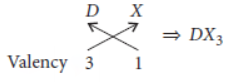
Let the element be D.
As D belongs to group 13, it will have valency = 3
Halide X has the valency = 1
So, chemical formula will be
(iii) Compounds formed when element of group-2
combines with an element of group-16 :
Let the group-2 element be X and group-16 element be Y.
Valency of X = 2
Valency of Y = 2
Chemical formula of the compound will be

Question. Study the following table in which positions of six elements A, B, C, D, E and F are shown as they are in the Modern Periodic Table :
On the basis of the above table, answer the following questions :

(i) Name the element which forms only covalent compounds.
(ii) Name the element which is a metal with valency three.
(iii) Name the element which is a non-metal with valency three.
(iv) Out of D and E, which is bigger in size and why?
(v) Write the common name for the family to which the elements C and F belong.
Answer: (i) Element E will form only covalent compounds because it has 4 electrons in the outermost shell so, it can neither loose nor gain 4 electrons, hence E forms compounds by sharing of electrons.
(ii) Element D is a metal having valency 3 as it belongs to group 13.
(iii) B is a non-metal with valency (8 – 5 =) 3.
(iv) Out of D and E, D will be bigger in size because as we move from left to right in a period there is addition of extra electron in the same shell due to which electrons are pulled more closer to the nucleus.
(v) C and F belong to group 18 and are called noble gases.
Question. Four elements P, Q, R and S belong to the third period of the Modern Periodic Table and have respectively 1, 3, 5 and 7 electrons in their outermost shells. Write the electronic configurations of Q and R and determine their valencies. Write the molecular formula of the compound formed when P and S combine.
Answer: P, Q, R and S all belong to 3rd period so, all of them will have 3 shells and the number of electrons in their outermost shell is 1, 3, 5 and 7 respectively.
∴ Electronic configuration of Q = 2, 8, 3 and its valency = 3
Similarly, electronic configuration of R = 2, 8, 5
and its valency = 8 – 5 = 3
Electronic configuration of P = 2, 8, 1
Thus, valency of P = 1
Electronic configuration of S = 2, 8, 7
Thus, valency of S = 8 – 7 = 1
Molecular formula of the compound :
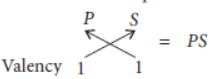
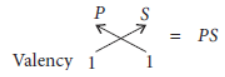
Question. (a) Two elements M and N belong to groups I and II respectively and are in the same period of the periodic table. How do the following properties of M and N vary?
(i) Sizes of their atoms.
(ii) Their metallic characters.
(iii) Their valencies in forming oxides.
(iv) Molecular formula of their chlorides.
Answer: (a)
Question. (a) How many periods are there in the Modern Periodic Table of elements?
(b) How do atomic radius, valency and metallic character vary down a group?
(c) How do the atomic size and metallic character of elements vary as we move from left to right in a period?
Answer: Valency remains the same in a group, as the number of valence electrons are same. Valency first increases from 1 to 4 in a period and then decreases to 0.
Question. What is meant by ‘group’ in the Modern Periodic Table? How do the following change on moving from top to bottom in a group?
(i) Number of valence electrons.
(ii) Number of occupied shells.
(iii) Size of atoms.
(iv) Metallic character of elements.
(v) Effective nuclear charge experienced by valence electrons.
Answer:The vertical columns in the Modern periodic table are called groups. There are total 18 groups in the Modern periodic table.
(i) In a particular group, the number of valence electrons remains the same.
(ii) On moving down the group, there is addition of an extra shell successively. Hence, number of occupied shells increases.
(iii) Due to addition of extra shells down the group, the size of the atoms i.e., the distance between nucleus and the outermost shell also increases.
(iv) Down the group as atomic size increases, the outermost electron is pulled by nucleus to lesser
extent and hence, tendency to loose electrons increases i.e., metallic character increases.
(v) Effective nuclear charge experienced by valence electrons decreases down the group due to increase in size of atoms.
Question. Write the number of groups and periods in the Modern Periodic Table. Mention the criteria of placing elements in the (i) same group and (ii) same period. Illustrate your answer with an example for each case.
Answer: There are 18 groups and 7 periods in the Modern periodic table.
(i) For elements to be in the same group, they should have same number of electrons in their outermost shells. For example, sodium and potassium have one electron in their outermost shells, so they belong to same group i.e., group 1.
(ii) For elements to be in the same period, they should have same number of shells.
For example, magnesium (12) and aluminium (13) contain three shells so, they belong to period 3.
Mg (12) = 2, 8, 2] Both have three shells
Al (13) = 2, 8, 3
Question. The atomic number of an element X is 19.
(a) Write its electronic configuration.
(b) To which period of the Modern Periodic Table does it belong and what is its valency?
(c) If ‘X’ burns in oxygen to form its oxide, what will be its nature – acidic, basic or neutral?
(d) Write balanced chemical equation for the reaction when this oxide is dissolved in water.
Answer: Atomic number of X = 19
(a) Electronic configuration of X = 2, 8, 8, 1
(b) X has four shells so, the period number of X = 4. Moreover, it has one electron in its outermost shell, so the valency of X will be equal to one.
(c) Electronic configuration of X shows that it is a metal and metals form basic oxides.
(d) When oxide of X is dissolved in water then its hydroxide will be formed.
X2O + H2O → 2XOH
Question. Study the following table in which positions of six elements A, B, C, D, E and F are shown as they are in the Modern Periodic Table :
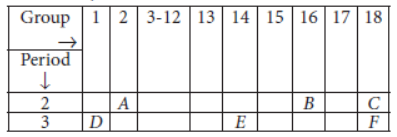
On the basis of the above table, answer the following questions :
(i) Name the element which will form only covalent compounds.
(ii) Which element is a metal with valency one?
(iii) Which element is a non-metal with valency two?
(iv) Out of D and E, which has a bigger atomic radius and why?
(v) Write the formula of the compound formed when B combines with D.
Answer: (i) Element E will form only covalent compounds.
(ii) Element D is a metal with valency one as it belongs to group 1.
(iii) Element B is a non-metal with valency 2 as it belongs to group 16 (valency = 8 – 6 = 2).
(iv) Out of D and E, D will have bigger atomic radius because as we move along the period from left to right there is decrease in atomic radius.
(v) Valency of B = 2 Valency of D = 1
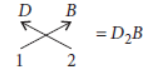
Question. The electronic configuration of an element is 2, 8, 8, 1.
(i) State its group number and period number in the Modern Periodic Table.
(ii) State whether this element is a metal or a non-metal.
Give reason for the justification of your answer in each case.
Answer: Electronic configuration of element = 2, 8, 8, 1
(i) It contains one electron in its outermost shell thus, it belongs to group 1. Moreover, the element has 4 shells, so it belongs to period 4.
(ii) As the element contains one electron in its outermost shell which can be easily lost hence, it acts as a metal.
Question. Given below are some elements of the Modern Periodic Table :
4Be, 9F, 14Si, 19K, 20Ca
(i) Select two elements that belong to the same period. Which one of the two has bigger atomic size?
Answer: (i) Be and F belong to the same period (period 2). K and Ca belong to the same period (period 4). Among Be and F, Be will be bigger in size and among K and Ca, K will be bigger in size.
Question. An element ‘X’ belongs to the third period and group one of the Modern Periodic Table.
Find (i) the number of its valence electrons (ii) its valency, and (iii) whether X is a metal or a non-metal. State reasons to justify your answer in each case.
Answer: As element X belongs to group 1, thus it will have one electron in its outermost shell. Moreover, it belongs to period 3 which implies that X has 3 shells.
(i) Electronic configuration of X will be 2, 8, 1
Hence, number of valence electrons = 1
(ii) Valency of X will be 1.
(iii) As X contains 1 valence electron which can be easily lost hence, it is a metal.
Question. F, Cl and Br are the elements each having seven valence electrons. Which of these (i) has the largest atomic radius, (ii) is most reactive? Justify your answer stating reason for each.
Answer: (i) F, Cl and Br all have seven valence electrons so, they belong to the same group. On moving down the group, the atomic size of the elements increases due to addition of extra shell at each successive element. Due to this the average distance between nucleus and outermost electrons increases. Thus, Br is largest in size among F, Cland Br.
(ii) Fluorine is the most reactive element because the chemical reactivity of non-metals decreases on going down a group as the size of the atoms goes on increasing. Hence, the attraction of incoming electrons decreases. Therefore, the tendency of atoms to gain electrons decreases due to which their reactivity decreases.
Question. (a) How will the tendency to gain electrons change as we go from left to right across a period? Why?
Answer: (a) endency to gain electrons increases as we go from left to right in a period due to addition of extra electron in the same shell at each successive element. Hence, tendency to attain a noble gas configuration also increases. Moreover, as the number of electrons increases in outermost shell there is an increase in effective nuclear charge due to which tendency to gain electrons increases.
Question. The elements Be, Mg and Ca each having two electrons in their outermost shells are in periods 2, 3 and 4 respectively of the Modern Periodic Table. Answer the following questions, giving justification in each case :
(i) Write the group to which these elements belong.
(ii) Name the least reactive element.
(iii) Name the element having largest atomic radius.
Answer: (i) As Be, Mg and Ca have two electrons in their outermost shell so, they all belong to group 2.
(ii) Be will be least reactive element, as down the group the reactivity of the elements increases.
Be being smaller in size as compared to others will have less tendency to loose electrons and hence, is less reactive.
(iii) As we move down the group, atomic radius increases hence, calcium will have the largest atomic radius.
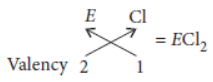
Question. The atomic number of an element is 16. Predict
(i) the number of valence electrons in its atom
(ii) its valency
(iii) its group number
(iv) whether it is a metal or a non-metal
(v) the nature of oxide formed by it
(vi) the formula of its chloride.
Answer: Atomic number of element (E) = 16
∴ Electronic configuration = 2, 8, 6
(i) Number of valence electrons in the atom = 6
(ii) Valency = 8 – 6 = 2
(iii) As there are 6 valence electrons thus, its group number is 10 + 6 = 16
(iv) This element is a non-metal.
(v) The nature of oxide formed by this element is acidic.
(vi) The formula of the chloride of non-metal ‘E’ will be
Question. The positions of three elements A, B and C in the periodic table are indicated below :
Group 16 Group 17
– – (First period)
– A (Second period)
– – (Third period)
B C (Fourth period)
(a) State whether element C would be a metal or a non-metal? Why?
(b) Which is the more active element A or C? Why?
(c) Which type of ion (cation or anion) will be formed by the element C? Why?
Answer: (a) C belongs to group 17 and hence, it will have 7 valence electrons in the outermost shell and has a tendency to gain electrons thus, it is a non-metal.
(b) Among A and C, A will be more reactive as the reactivity decreases down the group. So, A has more tendency to gain electrons.
(c) C will form negatively charged ion which is known as anion because group 17 elements have seven electrons in their outermost shell so, they have strong tendency to gain an electron to attain the noble gas configuration.
Question. Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electronic shells but different number of electrons in their outermost shell. It was found that elements A and G combine to form an ionic compound. This compound is added in a small amount to almost all vegetable dishes during cooking. Oxides of elements A and B are basic in nature while those of E and F are acidic. The oxide of D is almost neutral.
Based on the above information answer the following questions :
(i) To which group or period of the periodic table do the listed elements belong?
(ii) What would be the nature of compound formed by a combination of elements B and F?
(iii) Which two of these elements could definitely be metals?
(iv) Which one of the eight elements is most likely to be found in gaseous state at room temperature?
(v) If the number of electrons in the outermost shell of elements C and G be 3 and 7 respectively, write the formula of the compound formed by the combination of C and G.
Answer: Eight elements A, B, C, D, E, F, G and H have same number of electronic shells. So, they belong to the same period.
The biggest hint in the question is that the compound formed when A and G combine is used in almost all vegetable dishes which is NaCl.
Thus, A = Na and B = Cl
(i) These elements belongs to period number 3.
Group :
1 2 13 14 15 16 17 18
A B C D E F G H
Na Mg Al Si P S Cl Ar
(ii) The compound formed by the combination of B and F i.e., Mg and S will be ionic in nature as the bond will be formed by complete transfer of electrons.
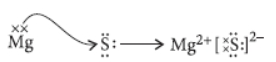
(iii) A and B i.e., sodium and magnesium will definitely be metals.
(iv) G i.e., Cl (chlorine) is found as gaseous diatomic (Cl2) molecule at room temperature.
(v) Number of electrons in outermost shell of C = 3
Number of electrons in outermost shell of G = 7
∴ Valency of C = 3
Valency of G = 8 – 7 = 1
Thus, the formula of the compound will be
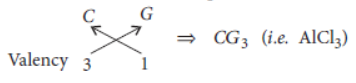
Question. In the following table six elements A, B, C, D, E and F (here letters are not the usual symbols of the elements) of the Modern Periodic Table with atomic number 3 to 18 are given :
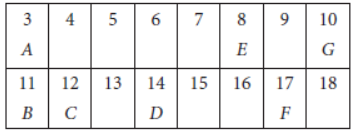
(a) Which of these (i) a noble gas, (ii) a halogen?
(b) If B combines with F, what would be the formula of the compound formed?
(c) Write the electronic configurations of C and E.
Answer: (a) (i) Noble gas = G
(ii) Halogen = F
(b) B(11) = 2, 8, 1
F(17) = 2, 8, 7
Valency of B = 1
Valency of F = 8 – 7 = 1
Formula of the compound formed :

(c) Electronic configuration of C(12) = 2, 8, 2
Electronic configuration of E(8) = 2, 6
Question. What are groups and periods in the periodic table? Two elements X and Y belong to group 1 and 2 respectively and are in the same period of the periodic table. How do the following properties of X and Y vary?
(i) Size of their atoms.
(ii) Their metallic character.
(iii) Their valencies in forming oxides.
(iv) Molecular formula of their chlorides.
Answer: The horizontal rows of elements in the periodic table are called periods. There are seven periods in the long form of periodic table.
The vertical columns in a periodic table are called groups. There are 18 groups in the long form of periodic table.
X belongs to group-1 while Y belongs to group-2 of the same period hence, valency of X will be 1 and valency of Y will be 2.
(i) As we move along the period from left to right the size of the atoms decreases. Hence, X will be bigger than Y.
(ii) Across the period from left to right, the metallic character decreases. Hence, X is more metallic than Y.
(iii) The valency of X in its oxide will be 1 and that of Y in its oxide will be 2.
(iv) Molecular formula of their chlorides will be
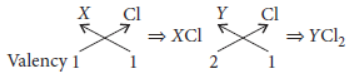
Question: Name the element which has
a. the electronic configuration 2, 8, 1.
b. a total of two shells, with 4 electrons in the valence shell.
c. a total of three shells, with 3 electrons in the valence shell.
d. one shell which is completely filled with electrons.
e. twice as many electrons in the second shell as in the first shell.
Answer: a. Sodium (2, 8, -1)
b. Carbon (2, 4)
c. Aluminium (2, 8, 3) i
d. Helium (2)
e. Carbon (2, 4)
Question: Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electronic shells but different number of electrons in their outermost shell. It was
found that elements A and G combine to form an ionic compound. This compound is added in a small amount to almost all vegetable dishes during cooking.
Oxides of elements A and B are basic in nature while those of E and F are acidic. The oxide of D is almost neutral. Based on the above information answer the following questions:
a. To which group or period of the Periodic Table do the listed elements belong to?
b. What would be the nature of the compound formed by the combination of elements ‘B’ and ‘F’?
c. Which two of these elements could definitely be metals?
d. Which one of the eight elements is most likely to be found in gaseous state at room temperature?
e. If the number of electrons in the outermost shell of elements ‘C’ and ‘G’ be 3 and 7 respectively, write the formula of the compound formed by the combination of‘C’and ‘G’.
Answer: a. A and B belongs to group-1 and group-2 respectively because they form basic oxides. ‘C’ belongs to group-13, ‘D’ belongs to group-14 which forms almost neutral oxide (actually amphoteric oxide), E and F belong to group-15, 16 forming acidic oxides. ‘G’ belongs to group-17 because NaCl is used in cooking. ‘H’ belongs to group 18.
they belong to third period of the periodic table.
b. B and F will form ionic compound because ‘B’ is a metal and ‘F’ is a non-metal.
c. A and B are definitely metals.
d. H is most likely to be found in gaseous state at room temperature.
e.
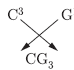
is the formula of compound.

