Please refer to the Periodic Classification of Elements Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 10th Science book. We have provided chapter wise Notes for Class 10 Science as per the latest examination pattern.
Revision Notes Chapter 5 Periodic Classification of Elements
Students of Class 10 Science will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 10 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
Doberiener’s Triads and Newlands Law of Octaves
There are 118 elements that are known at present. Some elements have similar properties whereas some others have completely contrasting properties.
You must have observed that in a grocery store, things are kept in an orderly manner. For example, soaps are stacked at one place while biscuits are kept separately at another place.
Scientists too tried to arrange elements based on their properties. However, as more and more elements were discovered, it became increasingly difficult to arrange these elements.
Hence, scientists began to look for some pattern in the properties of these elements. Let us study in this part how famous scientists such as Johann Wolfgang Dobereiner and John Newlands arranged the elements discovered at that time.
In 1829, Johann Wolfgang Dobereiner, a German chemist, classified elements into groups based on their properties. He kept all elements having similar properties in one group.
Most of his groups had three elements each. Thus, he called these groups as triads. He was the first person to illustrate the relationship between the atomic masses of elements and their properties.
Mass number is the sum of the number of protons and neutrons in an element.
He also gave a law known as the Law of Triads. It states that when three elements in a triad are listed in the increasing order of their atomic masses, the atomic mass of the middle element will roughly be the average of the atomic masses of the other two elements. This is demonstrated in the following animation.
The above example illustrates Dobereiner’s Law of Triads.
Similarly, this law can also be proved for the triads of other elements as shown below:
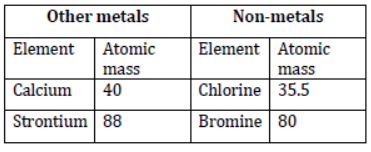

Average mass of calcium and barium = 88.5
Average mass of chlorine and iodine = 35.5+127 /2 = 81.2
In both cases, the average atomic mass of the middle element is approximately equal to the average atomic mass of the other two elements.
Hence, Dobereiner was able to identify only three triads from the elements known at that time as shown in table 1.
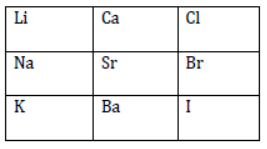
Table 1: Dobereiner’s triads
Limitations of Dobereiner’s classification of elements:
- All known elements could not be classified into groups of triads on the basis of their properties.
- Not all groups obeyed the Law of Triads. For example, nitrogen family does not obey the Law of Triads.

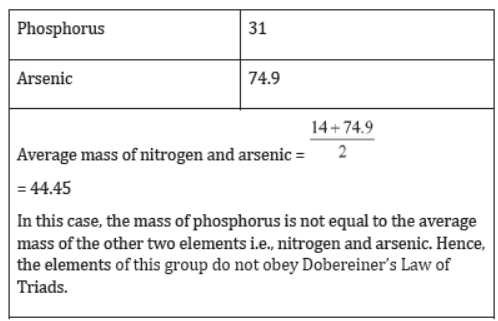
Although Dobereiner tried to classify elements into groups based on their properties, his classification was not very useful.
In 1866, John Newlands, an English scientist, arranged the known elements in an increasing order of their atomic masses.
He began with hydrogen, which has the lowest atomic mass. He observed that if the elements are arranged in the increasing order of their atomic masses, then every eighth element (starting from a given element) had properties similar to those of the first element. Therefore, he arranged 56 elements in seven groups such that elements having same properties were present below each other in the form of a group. A part of the original Newlands’ Octaves is shown in table 2.

Table 2: Newlands’ Octaves
He compared his table to the octaves of music. It is for this reason that he called his model the Law of Octaves or Newlands’ Law of Octaves.
DO YOU KNOW?
There are seven musical notes in the Indian system of music. They are – sa, re, ga, ma, pa, da, ni. Similarly, in the west, they use the notations – do, re, mi, fa, so, la, ti. Every eighth note is similar to the first one and it is the first note of the next scale.
According to Newlands’ Law of Octaves, the properties of fluorine (eighth element starting from hydrogen) are similar to those of hydrogen. Similarly, the properties of sodium are similar to those of lithium; the properties of magnesium are similar to those of beryllium; and so on.
Newlands’ arrangement of atoms showed for the first time that elements could be arranged and grouped based on some fundamental property such as the atomic mass.
Do you know that Newlands’ Law of Octaves was found applicable only for the elements having low atomic masses?
Newlands’ Law of Octaves has many limitations, which are discussed below.
- This law was not applicable throughout the arrangement. It was applicable only till calcium.
- Newlands’ assumed that only 56 elements would exist in nature and believed that no more elements would be discovered. However, several elements were discovered in the following years. These elements did not follow the Law of Octaves.
- The positions of cobalt and nickel could not be explained according to Newlands’ Law of Octaves. He kept cobalt and nickel in the same slot. They were also placed in the same column as fluorine and chlorine, which have completely different properties.
- The properties of iron are similar to those of cobalt and nickel. However, iron was placed away from them in a different column.
Mendeleev’s Periodic Table
In 1866, John Newlands, an English scientist, arranged the known elements in an increasing order of their atomic masses, and his model is known as Newlands’ Law of Octaves. Newlands began with hydrogen, which has the lowest atomic mass. He observed that if elements were arranged in the increasing order of their atomic masses, then every eighth element (starting from a given element) had properties similar to those of the first element. Therefore, he arranged the elements in seven groups such that elements having the same properties were present below each other in the form of a group. However, Newlands’ Law of Octaves has many limitations. Hence, it was not highly accepted.
Limitations of Newlands’ Law of Octaves:
It was not applicable throughout the arrangement. It was applicable only till calcium.
- Newlands assumed that only 56 elements would exist in nature and believed that no more elements would be discovered. However, several elements were discovered in the following
years.
- The positions of cobalt and nickel could not be explained according to Newlands Law of Octaves. They were placed in the same column below fluorine and chlorine, which have completely different properties.
- The properties of iron are similar to those of cobalt and nickel. However, iron was placed away from them in a different group.
Lother Meyer’s arrangement of elements
In 1869, a German chemist, Lother Meyer plotted the different physical properties of elements, like atomic volume, density, melting point, boiling point, thermal conductivity etc. against their atomic weights. He found that these properties varied in a periodic fashion. Based on this, he arranged elements in a way which later nearly resembled Mendeleev’s arrangement of elements.
After the failure of Newlands Law of Octaves, scientists continued to correlate the properties of elements with their atomic masses. Dmitri Ivanovich Mendeleev (1834- 1907), a Russian scientist, also tried to relate the properties of elements with their atomic masses.
Mendeleev was the first person to introduce the concept of a periodic table. His periodic table was based on the atomic mass as well as the properties of elements. He arranged elements in the increasing order of their atomic masses and grouped the elements having similar properties together. Mendeleev formulated the periodic law in 1869.
Mendeleev’s Periodic Law:
This law states that the properties of elements are a periodic function of their atomic masses. This means that arrangement of elements in the increasing order of their atomic masses, results in repetition of their properties after regular intervals.
Only 63 elements were known when Mendeleev first started his work. He arranged these elements in the form of a table in the increasing order of their atomic masses. This table is known as Mendeleev’s periodic table as shown in the table. His table contains vertical columns called ‘groups’ and horizontal rows called ‘periods’.
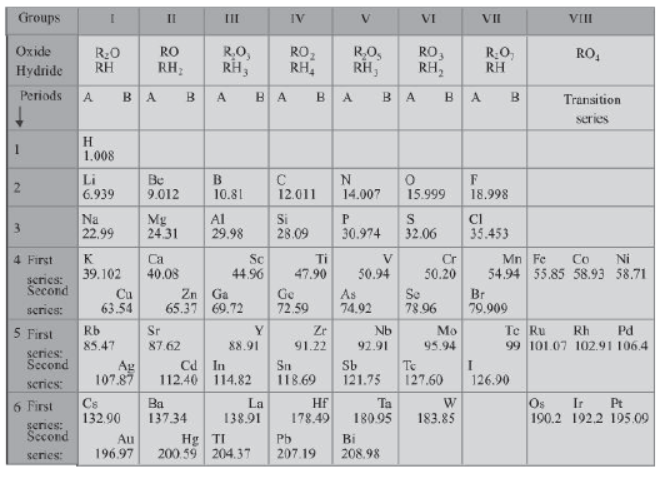
Do you know that Mendeleev’s periodic table was a huge success? Let us now study the achievements of Mendeleev’s periodic table.
Achievements of Mendeleev’s periodic table:
- Mendeleev left some gaps in his periodic table. He had predicted that some elements were yet to be discovered. He left these gaps deliberately so that these undiscovered elements could get a place in his periodic table.
- Mendeleev named the undiscovered elements using the Sanskrit word Eka (meaning one) as a prefix, with the name of the preceding element in the same group. For example, gallium was not discovered in Mendeleev’s time. Therefore, he left a gap for it in his periodic table and named it Eka-aluminium. He also predicted the properties of these undiscovered elements based on their positions in the periodic table. A comparison of the properties of gallium as predicted by Mendeleev and its actual properties is given in the following table.
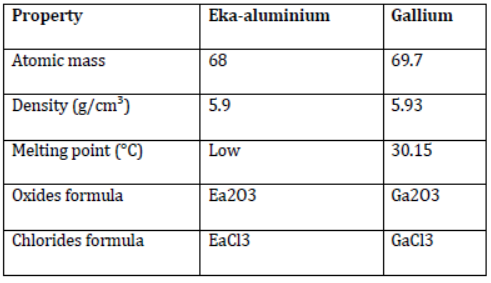
Comparison of the properties of Eka-aluminium and gallium
In the periodic table, Ea is used as the symbol for Eka-aluminium. It can be clearly observed from the table that Mendeleev’s predictions were almost exact. This extraordinary achievement made Mendeleev’s periodic table very popular.
- Noble gases were not discovered at the time when Mendeleev gave the periodic table. These were discovered in recent times as they are very inert and are present in very low concentrations in the atmosphere. When these gases were finally discovered, they got a place in his periodic table as a separate column. The accommodation of these gases in the periodic table did not disturb the positions of other elements. This underlined the strength of Mendeleev’s periodic table.
- Although Mendeleev arranged the elements in the increasing order of their atomic masses, there were instances where he placed an element with a slightly higher atomic mass before an element with a slightly lower atomic mass.
For example, cobalt, whose atomic mass is 58.9, was placed before nickel whose atomic mass is 58.7. This was done to maintain consistency in the properties of the elements present in a group i.e., to group the elements with similar properties together.
Though, Mendeleev’s periodic table was a huge success at that time, it still had many limitations. Now, let us discuss the limitations of Mendeleev’s periodic table.
Limitations of Mendeleev’s periodic table:
Hydrogen’s position was not justified in Mendeleev’s periodic table. He positioned hydrogen in the first column above alkali metals. He did so because hydrogen and alkali metals have similar properties. For example, hydrogen reacts with halogens, oxygen, and sulphur to form compounds whose formulae are similar to those of alkali metals.

Hydrogen and alkali metals reacting with halogens
However, hydrogen also resembles halogens in many ways. Like halogens, hydrogen is a gas, and exists as a diatomic molecule (H2). It forms covalent compounds like halogens unlike alkali metals. Hence, it can also be placed above the halogen group.
Therefore, Mendeleev was not able to explain the position of hydrogen. In other words, the position of hydrogen in Mendeleev’s periodic table was not justified. This was the first limitation of Mendeleev’s periodic table.
2.The discovery of isotopes revealed another limitation of Mendeleev’s periodic table.
Since Mendeleev’s periodic table was based on atomic masses of elements, isotopes should be placed in different columns despite the fact that they represent the same element.
Atoms of the same elements having different number of neutrons are called isotopes. Isotopes have the same number of protons and electrons, but different number of neutrons. For example, the isotopes of chlorine are Cl-35 and Cl-37. They have the same atomic number, but different atomic masses.
Modern Periodic Table
Mendeleev made a successful effort in grouping elements in the form of his periodic table. He had many achievements, but there were many limitations in his Periodic Table as well.
Some limitations of Mendeleev’s periodic table are listed below:
The position of hydrogen was not justified in Mendeleev’s periodic table.
The discovery of isotopes revealed another limitation of Mendeleev’s periodic table.
Although Mendeleev arranged the elements in the increasing order of their atomic masses, there were instances where he had placed an element with a slightly higher atomic mass before an element with a slightly lower atomic mass.
The limitations of Mendeleev’s periodic table forced scientists to believe that atomic mass could not be the basis for the classification of elements.
In 1913, Henry Moseley demonstrated that atomic number (instead of atomic mass) is a more fundamental property for classifying elements. The atomic number of an element is equal to the number of protons present in an atom of that element. Since the number of protons and electrons in an atom of an element is equal, the atomic number of an element is equal to the number of electrons present in a neutral atom.
Atomic number = Number of protons = Number of electrons
The number of protons or electrons in an element is fixed. No two elements can have the same atomic number. Hence, elements can be easily classified in the increasing order of their atomic numbers. In the light of this fact, Mendeleev’s Periodic Law was done away with. As a result, the modern periodic law came into the picture.
The modern periodic law states that the properties of elements are a periodic function of their atomic numbers, not their atomic masses.
The table that is obtained when elements are arranged in the increasing order of their atomic numbers is called the Modern Periodic Table or Long Form of the Periodic Table as shown in the figure.

The Modern periodic table
In the modern periodic table, the elements are arranged in rows and columns. These rows and columns are known as periods and groups respectively. The table consists of 7 periods and 18 groups.
Do You Know:
In the modern periodic table, hydrogen is placed above alkali metals because of resemblance with their electronic configurations. However, it is never regarded as an alkali metal. This makes hydrogen a unique element.
If you look at the modern periodic table, you will find that all elements in the same group contain the same number of valence electrons. Let us see the following activity to understand better.
Activity 1: Look at group two of the modern periodic table. Write the name of the first three elements followed by their electronic configurations.
What similarity do you observe in their electronic configurations? How many valence electrons are present in these elements?
The first three elements of group two are beryllium, magnesium, and calcium. All these elements contain the same number of valence electrons. The number of valence electrons present in these elements is 2. On the other hand, the number of shells increases as we go down the group.
Again, if you look at periods in the modern periodic table, you will find that all elements in the same period contain the same valence shell. Let us see the following activity to understand better.
Activity 2: Look at the elements of the third period of the modern periodic table. Write the electronic configuration of each element and calculate the number of valence electrons present in these elements.
What do you observe from the given activity? Do these elements contain the same number of shells? How many valence electrons are present in these elements?
You will find that elements such as sodium, magnesium, aluminium, silicon, phosphorus, sulphur, chlorine, and argon are present in that period. The valence shell in all these elements is the same, but they do not have the same number of valence electrons.

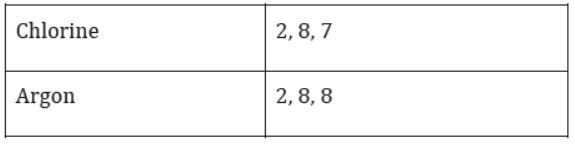
Thus, the number of electrons in the valence shell increases by one unit as the atomic number increases by one unit on moving from left to right in a period.
Let us calculate the number of elements that are present in the first, second, third, and fourth periods.
The maximum number of electrons that a shell can hold can be calculated using the formula 2n2. Here, n represents the number of shells from the nucleus. For example, n is equal to 1, 2, and 3 for K, L, and M shells respectively. Hence, the maximum number of electrons that each of these shells can hold can be calculated by substituting the value of n in the given formula.
Number of electrons that K shell can accommodate = 2n2
= 2 x 12
= 2
Hence, K shell can accommodate only 2 electrons and only two elements are present in the first period.
Similarly, the second and third shell (L and M respectively) can accommodate 8 and 18 electrons respectively. Since the outermost shell can contain only 8 electrons, there are only 8 elements in both the periods.
Important Note:
The position of an element in the Modern Periodic Table tells us about its chemical reactivity. The valence electrons determine the kind and the number of bonds formed by an element.
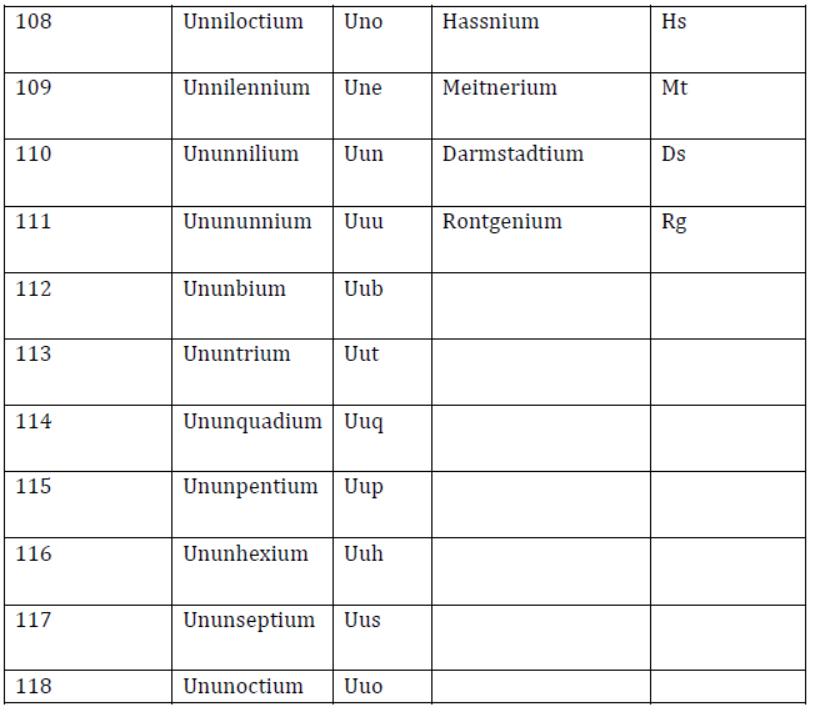
IUPAC Nomenclature for Elements with Atomic Number > 100
- Latin word roots for various digits are listed in the given table.
Notation for IUPAC Nomenclature of Elements

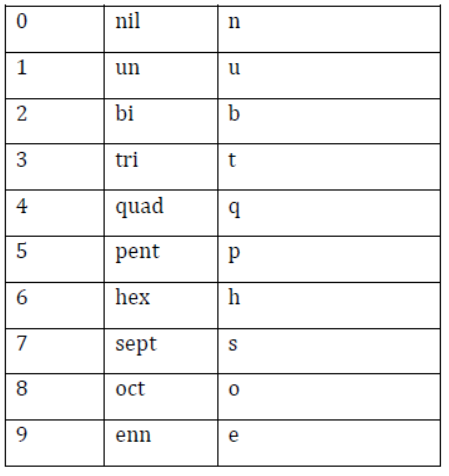
- Latin words for various digits of the atomic number are written together in the order of digits, which make up the atomic number, and at the end, ‘ium’ is added.
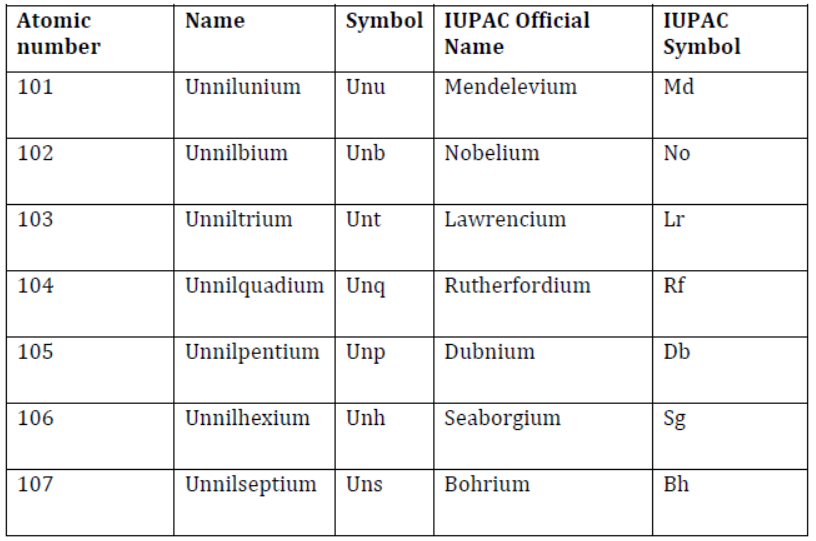
- Nomenclature of elements with the atomic number above 100 is listed below.
Nomenclature of Elements with Atomic Number Above 100
Trends in the Modern Periodic Table
If you observe the long form of the periodic table or the modern periodic table, then you will find that there are certain trends followed. These trends help us in determining various properties of the elements. For example, elements like sodium, magnesium, aluminium, silicon, phosphorus, sulphur, chlorine, and argon are present in the third period. Do you know why they are placed like this?
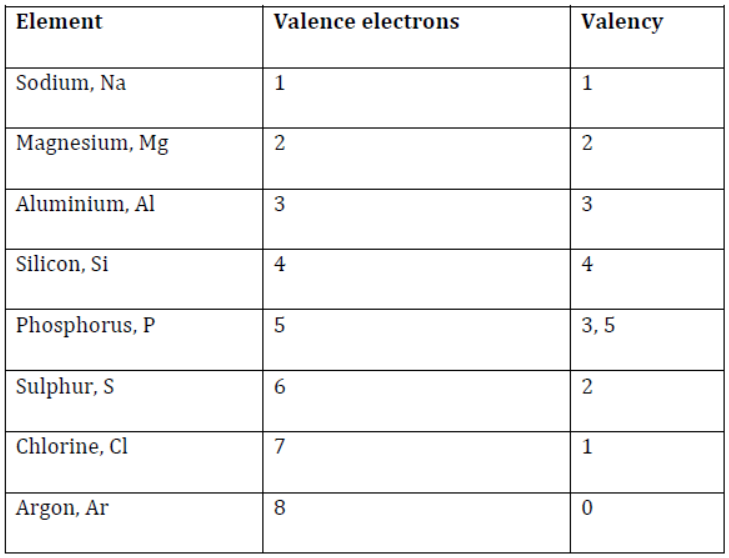
It is because all these elements have one thing in common. You must have observed that these elements contain the same number of shells, and the last electron enters shell M as shown in the given table:
Elements of the third period


Do you know the valency of the elements present in the third period?
Valency is defined as the number of electrons an atom requires to lose, gain, or share in order to complete its valence shell to attain the stable noble gas configuration. Valencies of the elements can also be determined by the number of electrons present in the outermost shell known as the valence shell.
Therefore, on moving across a period, from left to right, the valency first increases from 1 to 4, and then decreases from 4 to 0.
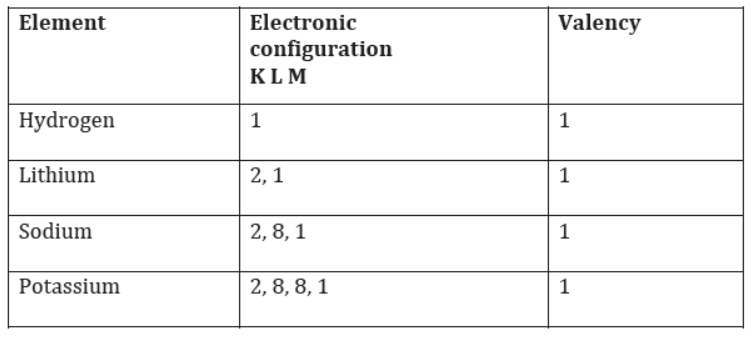
Do you know how the valency of an element, present in the same group, changes on moving down the group?
The number of valence electrons present in atoms of elements belonging to a particular group remains the same. As a result, valency also remains the same. Hence, the valency of group 1 elements is one, group 2 elements is two, and so on.
For example, the valency of the first four elements of group 1 of the periodic table is given as follows. The valency of all these four elements is 1.
Valency of the first four elements of group 1
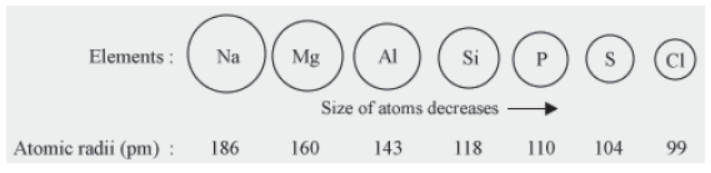
Atomic size is the radius of an atom in its neutral state i.e. the distance of the nucleus from its valence shell in an isolated gaseous atom.
Do you know how atomic size changes as we move from top to bottom or across the periodic table. Let us see.

Changes in atomic size on moving down a group
The atomic radii of the elements present in the third period are given in Pico meters.
Changes in atomic size on moving across a period
Do you know that the metallic character of elements can also be determined with the help of the periodic table?
Metals are electropositive in nature as they lose electrons to form positive ions called cations. For example, Na loses one electron to form Na+ ion.
We have learnt that there is a decrease in the atomic size, as we move across a period. As a result, it becomes difficult for an atom to lose electrons as we move from left to right across the periodic table. Hence, metallic character decreases on moving across a period from left to right.
On the other hand, atomic size increases when we move down a group. This makes it easier for an element to lose electrons. Hence, metallic character increases on moving down a group.
Therefore, the elements present in the 1st group are the most metallic in their respective periods as they contain only one electron, and have the largest atomic size in their respective periods.
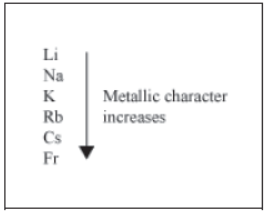
Changes in metallic character on moving down a group
Do you know that with the help of a periodic table, we can also predict the nature of the oxides formed by the various elements? The oxides formed by metals are basic in nature, whereas the oxides of non-metals are acidic in nature. Hence, the elements present on the left hand side of the periodic table will form basic oxides, whereas the elements present on the right hand side of the periodic table will form acidic oxides.
Elements show periodicity because of their valence shell configuration. All elements showing periodicity in properties have the same number of electrons in the last or valence shell. The properties that will be discussed here are:
- Atomic radius
- Ionisation potential
- Electron affinity
- Electronegativity
- Metallic character
Atomic Radius: The atomic radius is usually considered as the distance from the centre of the nucleus to the outermost shell i.e., to a point where the electron density is effectively zero.
Across the period i.e., from left to right: Atomic radius decreases
Down the group i.e., from top to bottom: Atomic radius increases
Reason: Across the period, the effective nuclear charge increases. This is due to the fact that the number of electrons increases (in the same subshell), and so does the number of protons in the nucleus. This pulls the valence shell of electrons in an atom towards itself, thus decreasing the atomic radius. But as we move down the group, the number of shells keeps on increasing along with the number of protons. The space required to accommodate the extra shells becomes the dominating factor and therefore the atomic size increases.
Ionisation Potential: It is the energy required to remove one mole of electrons from the valence shell of one mole of isolated gaseous atoms.
Across the period i.e., from left to right: Ionisation potential increases
Down the group i.e., from top to bottom: Ionisation potential decreases
Reason: Across the period, the effective nuclear charge increases. This causes the atomic radius to decrease, thus getting the valence shell closer to the nucleus. This makes it difficult to remove electrons. But as we move down the group, the number of shells keeps on increasing along with the number of electrons. The distance from the nucleus coupled with the interference of the electron between the nucleus and the valence shell renders the valence electrons weakly bound to the nucleus.
Electronegativity: The tendency of an atom of an element to attract a shared pair of electrons towards itself when bonded with another element in a compound is called electronegativity.
Across the period i.e., from left to right: Electronegativity increases
Down the group i.e., from top to bottom: Electronegativity decreases
Reason: Across the period, the effective nuclear charge increases, thus decreasing the atomic radius. This favours the increase in electronegativity of elements across the period. But as we move down the group, the number of shells keeps on increasing and therefore the atomic size increases and the electronegativity decreases.
Metallic character: It is defined as the tendency of an atom to lose electrons.
Across the period i.e., from left to right: Metallic character decreases
Down the group i.e., from top to bottom: Metallic character increases
Reason: Across the period, the effective nuclear charge increases, thus decreasing its atomic radius. This favours the increase of electronegativity and therefore the tendency to lose electrons is low. This accounts for the decrease in the metallic character along a period. But as we move down the group, the number of orbits keeps on increasing and therefore the atomic size increases. This means that the electronegativity decreases. This enhances the loss of electrons and therefore the metallic character increases down a group.



