Please see Chapter 5 Surface Chemistry Exam Questions Class 12 Chemistry below. These important questions with solutions have been prepared based on the latest examination guidelines and syllabus issued by CBSE, NCERT, and KVS. We have provided Class 12 Chemistry Questions and answers for all chapters in your NCERT Book for Class 12 Chemistry. These solved problems for Surface Chemistry in Class 12 Chemistry will help you to score more marks in upcoming examinations.
Exam Questions Chapter 5 Surface Chemistry Class 12 Chemistry
[SINGLE CORRECT CHOICE TYPE]
Question. Which gas will be adsorbed on a solid to greater extent.
(A) A gas having non polarmolecule
(B) A gas having highest critical temperature (Tc)
(C) A gas having lowest critical temperature
(D) A gas having highest critical pressure
Answer
B
Question. Which of the following factors affects the adsorption of a gas on solid?
(A) Tc(critical temp.)
(B) Temperature of gas
(C) Pressure of gas
(D) Allof them
Answer
D
Question. The volume of gases NH3, CO2 and CH4 adsorbed by one gram of charcoal at 298 Kare in
(A) CH4 > CO2 > NH3
(B) NH3 > CH4 > CO2
(C) NH3 > CO2 > CH4
(D) CO2 > NH3 > CH4
Answer
C
Question. The heat of physisorption lie in the range of
(A) 1 –10 kJ mol–1
(B) 20 to 40 kJ mol–1
(C) 40 to 200 kJ mol–1
(D) 200to 400 kJ mol–1
Answer
B
Question. Adsorption is multilayer in case of
(A) physical adsorption
(B) chemisorption
(C) in both
(D) none of the these
Answer
A
Question. Reversible adsorption is
(A) chemical adsorption
(B) physical adsorption
(C) both
(D) none
Answer
B
Question. Which of the following is not a gel?
(A) Cheese
(B) Jellies
(C) Curd
(D) Milk
Answer
D
Question. An emulsion is a colloidal system of
(A) two solids
(B) two liquids
(C) one gas and one solid
(D) one gas and one liquid
Answer
B
Question. Which of the following is a lyophobic colloid?
(A) Gelatin
(B) Sulphur
(C) Starch
(D) Gum
Answer
B
Question. The nature of bonding forces in adsorption
(A) purely physical such as VanDerWaal’s forces
(B) purely chemical
(C) both chemical and physical always
(D) none of these
Answer
D
Question. The Tyndall effect associated with colloidal particles is due to
(A) presence of electrical charges
(B) scattering of light
(C) absorption of light
(D) reflection of light
Answer
B
Question. Which one of the following is not applicable to chemisorption?
(A) Its heat of adsorption is high
(B) It takes place at high temperature
(C) It is reversible
(D) It forms mono-molecular layers
Answer
C
Question. In the colloidal state the particle size ranges
(A) below1 nm
(B) between 1 nm to 1000 nm
(C)more than 100 nm
(D) none of the above
Answer
B
Question. All colloids
(A) are suspensions of one phase in another
(B) are two-phase systems
(C) contain onlywater-soluble particles
(D) are true solutions
Answer
B
Question. Colloids can be purified by
(A) condensation
(B) peptization
(C) coagulation
(D) dialysis
Answer
D
CASE-BASED/PASSAGE-BASED INTEGRATED QUESTIONS
Read the following passage and answer the questions that follow:
1.Surface chemistry deals with phenomenon that occur at the surfaces. Corrosion electrode processes, heterogeneous catalysis, dissociation and crystallisation occur at the surface. Evaporation is a surface phenomenon. Adsorption takes place at the surface. Easily liquefiable gas are more easily adsorbed on the surface of catalyst. Gas mass contain activated charcoal to adsorb poisonous gases.
Question. Out of H2, N2, CO and NH3, which gas will absorb to maximum extent and why?
Answer. NH3, because it has high critical temprature maximum intermolecular forces of attraction easily liquefiable.
Question. Write the reaction at anode and cathode in corrosion of iron?
Answer. Fe(s) → Fe2+(aq) + 2e– (At anode)
4H+ + O2 + 4e– → 2H2O (At cathode)
Question. What happen when O2 is adsorbed on the metallic surface?
Answer. Heat is released, bond length of O2 is increased and p* 2p orbital of O2 is occupied.
Question. What will happen if aqueous solution of raw sugar is passed over activated charcoal and why?
Answer. It becomes colourless as the colouring material gets adsorbed on the surface of charcoal.
Question. What happens of Λm (molar conductivity) of an aqueous solution of soap (sodium stearate) of CMC (Critical Micelle Concentration)?
Answer. At CMC, the concentration of current carrier anions decreases, hence molar conductivity Λm decreases abruptly,
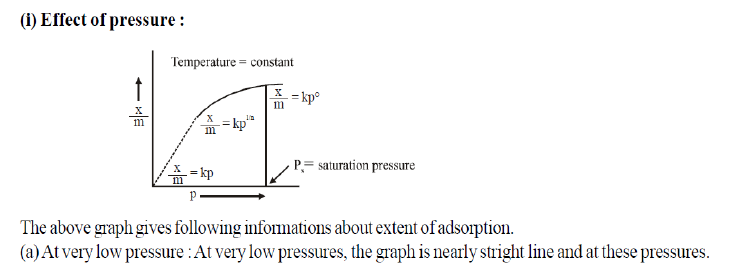
2.Observe the graph between logm/x (extent of adsorption) Vs log p(pressure) at constant temperature and answer the questions that follow:

Question. What does this graph shows?
Answer. It shows logm /x is directly proportional to log p i.e. extent of adsorption of gases on solid increase with increase in pressure.
Question. What is slope of line equal to?
Answer. It is equal to 1/n , where ‘n’ is whole number except zero, ‘ 1/n ’ lies between 0 and 1.
Question. What does intercept of line represent?
Answer. log k where ‘k’ is constant depending upon native of adsorbent and adsorbate.
Question. Write the expression of Freundlich adsorption isotherm of moderate pressure.
Answer. logm /x = log K + 1/n log p Or m /x = Kp1/n
Question.What is effect of temperature on chemisorption?
Answer. It first increases, then decreases with increase in temperature.
3. Observe the table 1, 2 in which coagulation values of cations and anions are given and table 3 given gold numbers of some protective colloids. Answer the questions based on these table.
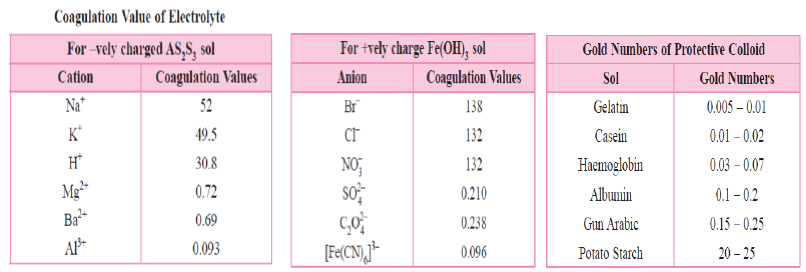
Question. Which ion has maximum coagulation power for As2S3 sol?
Answer. Al3+
Question. Which ion is best to coagulate Fe(OH)3 Fe3+ sol?
Answer. [Fe(CN)6]3–
Question. Which is best protective colloid?
Answer. Gelatin
Question. What is protective colloid?
Answer. It is lyophilic sol which stabilises lyophobic sols and prevents its coagulation.
Question. Which is least effective protective colloid?
Answer. Potato starch
ONE MARK QUESTION
Question. Howdoes chemical adsorption of a gas on a solid vary with temperature?
Answer. The rate of chemical adsorption first increases and then decreases as the temperature increases.
Question. Define Hardy-Schulze rule.
Hardy-Schulze rule states that
Answer. (i)The ions carrying charge opposite to that of sol particles are effective in causing the coagulation of the sol.
(ii)Coagulating power of an electrolyte is directly proportional to the fourth power of the valency of the ions causing coagulation.
Example : For negatively chargedAs2S3 sol, the positively charged Fe3+ ions are more effective than Ba2+ or Na+ ions.
Question. How are the colloids classified on the basis of the nature of interaction between dispersed phase and dispersion medium?
Answer. Depending upon the nature of interaction between the dispersed phase and the dispersionmedium,
colloids are divided into two categories.
(i)Lyophilic sols :The colloids in which the particles of the dispersed phase have a strong affinity for the dispersion medium are called lyophilic sols.These colloidal solutions, even if precipitated, change back to the colloidal form simply by adding dispersion medium. So lyophilic sols are reversible in nature e.g. glue, starch, rubber, etc.
(ii)Lyophobic sols :The colloids in which particles of the dispersed phase have no or very little affinity for dispersion medium are called lyophobic sols. These are irreversible in nature i.e. once precipitated ,they have little tendency to get back into the colloidal form on simply adding dispersion medium e.g. As2S3 sol.Lyophobic sols need stabilising agents for their preservation
Question. What happens when gelatin is added to gold sol?
Answer. Gold sol which is lyopobic sol starts behaving like a lyophilic colloid when gelatin is added to it.
Question. What causes Brownian movement in a colloidal solution?
Answer. The molecules of dispersion medium due to their kinetic motion strike against the colloidal particles (dispersed phase) from all sides with different forces causing them to move.
However, colloidal particles being comparatively heavier , move with slower speed.
Question. Which has a higher enthalpy of adsorption, physisorption or chemisorption?
Answer. Enthalpy of chemisorption is high (80 – 240 kJmol–1) as it involves chemical bond formation.
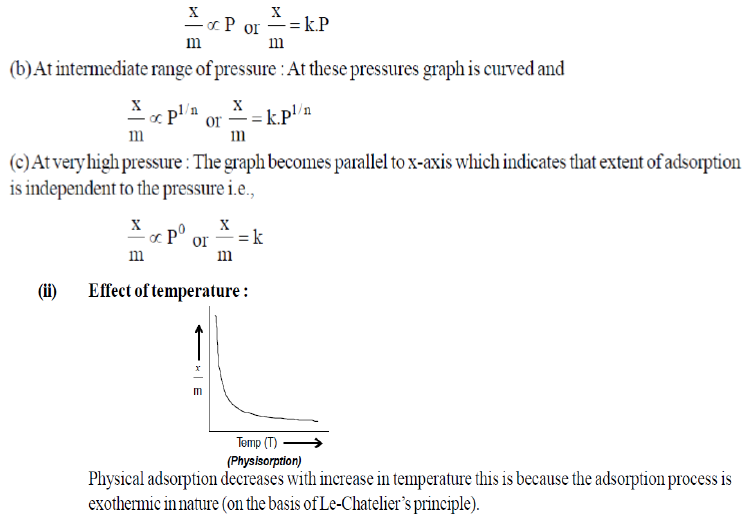
Question. Why does physisorption decrease with the increase of temperature?
Answer. Since adsorption process is exothermic and the physical adsorption occurs readily at low temperature and decreases with increase in temperature (Le-Chatelier’s principle).
Question. What is the basic difference between adsorption and absorption?
Answer. In adsorption, the substance is concentrated only at the surface and does not penetrate through the surface tothe bulk of the adsorbent,while in absorption, the substance is uniformly distributed throughout the bulk of the solid.
THREE MARK QUESTION
Question. Explain the following:
(a) Same substance can act both as colloids and crystalloids.
(b) Artificial rain is caused by spraying salt over clouds.
(c) When a beam of lights is passed through a colloidal sol, the path of the beam gets illuminated.
Answer. (a) The same substance can act as both colloid and crystalloid. It depends on the size of the particles. When the size of the particles lies between 1 to 1000 nm, it behaves as a colloid. If particle size is less than 1mm, it exists as a true solution and behaves like a crystalloid.
(b)Artificial rain is caused by spraying salt over clouds. The colloidal water particles of the clouds get neutralised by oppositely charged ions of the salt and coagulate to bigger water drops which cause artificial rain.
(c)When a beam of light is passed through a colloidal solution, the path of beam gets illuminated with visible light. This is due to scattering of light by colloidal particels (Tyndal effect)
Question. Explain what is observed when:
(i) An electrolyte, KCl, is added to hydrated ferric oxide sol
(ii) An electric current is passed through a colloidal solution.
(iii) A beam of strong light is passed through a colloidal solution.
Answer. (i)When an electrolyte like KCl is added of Fe(OH)3 sol, the positively charged colloidal particles of Fe(OH)3 get coagulated by the oppositely charged Cl– ions provided by KCl.
(ii) On passing the electric current, colloidal particles move towards the oppositely charged electrode where they lose their charge and get coagulated.
(iii) When a beam of strong light is passed through a colloidal solution scattering of light by colloidal particles takes place and the path of light becomes visible. This phenomenon is called Tyndall effect.
Question. Explain the following observations:
(i)A beam of light passing through a colloidal solution has a visible path
(ii)Passing an electric current through a colloidal solution removes colloidal particels from it.
(iii) Ferric hydroxide sol coagulates on addition of a solution of potassium sulphate.
Answer. (i) This is due to the scattering of light by colloidal solution.This effect is called Tyndal effect.
(ii) The charged colloidal particles move towards the oppositely charged electrodes on passing current Through the colloidal solution.
(iii) Ferric hydroxide sol is positively charged.When a solution ofK2SO4 is added to it, the sol gets coagulated by the negatively charged SO42– ions furnished by the added electrolyte.
Question. What are detergents? Give their scheme of classification. Why are the detergents preferred over soaps?
Answer. A detergent is a surface-active agent used for cleaning dirty surfaces. It contains a nonpolar hydrocarbon chain(hydrophobic part) and polar group (hydrophilicpart) with in the molecules and thus shows adsorption and micellization.
On the basis of charge on polar part, detergents are classified as follows :
(i)Anionic detergents in which large part of the molecule acts a anion e.g. alkyl benzene-sulphonate.
(ii)Cationic detergents which are most lyacetates or chlorides of quaternary amines e.g. Cetyltrimethyl ammoniumchloride.
(iii)Nonionic detergents like esters of high molecular mass formed by reaction between polyethylene glycol and stearic acid.
Detergents are preferred over soaps as they work even in hard water and acidic pH where soaps become in soluble. They have powerful cleansing action.
Question. Explain what is observed when
(i) An electrolyte is added to ferric hydroxide sol.
(ii) An emulsion is subjected to centrifugation.
(iii) Direct current is passed through a colloidal sol.
Answer. (i)When an electrolyte like NaCl is added to ferric hydroxide sol, the Cl– ions of NaCl neutralize the
positive charge of ferric hydroxide sol particles and coagulation of the sol take place.
(ii)When an emulsion is subjected ti centrifugation, its two liquids separate into different layers i.e. Demulsification occurs.
(iii)When an electric current is passed through a colloidal sol, its colloidal particles move towards oppositely charged electrodes, where they lose their charge and get coagulated.
Question. Define adsorption and write two important differences between physical adsorption and chemisorption.
Answer. Adsorption: It is the phenomenon of attracting and retaining the molecules of a substance on the surface of liquid resulting into a higher concentration on the surface.
Physical adsorption Chemisorption
(i) Forces of attraction between adsorbent (i) Forces between adsorbent and
adsorbate are weak Vander Wall’s forces. adsorbate are strong chemical forces.
(ii) Heat of adsorption is low (ii) Heat of adsorption is high
Question. Explain the following terms giving an example in each case.
(i) Electrophoresis (ii)Coagulation (iii) Dialysis
Answer. (i) Electrophoresis
It is the migration of electrically charged colloidal particles in one direction under the influence of an electric field. When colloidal particles move towards positive electrode, they are negatively charged and vice versa. Electrophoresis is used to measure the rate of migration of sol particles.
(ii) Coagulation
It is the precipitation of colloids by removal of the charge associated with the colloidal particles.Usually an electrolyte is added to affect coagulation. The sol can also be coagulated by boiling or freezing.
(iii) Dialysis
The process of removing a dissolved substance from the colloidal solution by means of diffusion through a suitable membrane is called dialysis. The apparatus used for this purpose is called dialyser (parchment paper or animal membrane).
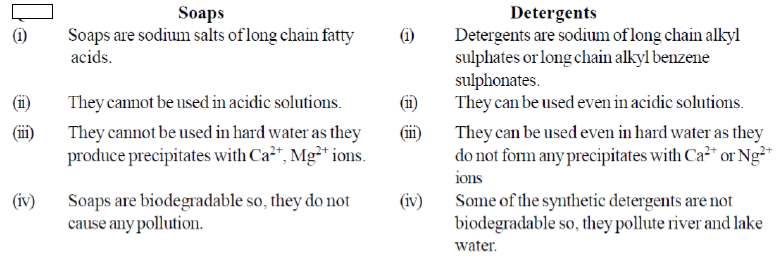
Question. Differentiate between soaps and detergents
Answer.
Question. What are the two classes of emulsions? give one example of each class. State on activity to test the type of an emulsion.
Answer. The two types of emulsions are :
(i)Oil-in-water type in which small droplets of an oil are dispersed in water.for example milk, cod liver oil.
(ii)Water-in-oil type in which water droples are dispersed in an oil medium.for example, butter.
Dye test : Some oil soluble dye is added to the emulsion. If the background becomes coloured, the emulsion is water-in-oil type and if the coloured droplets are seen, then the emulsion is oil-inwater type.
Question. Explain what is observed when
(a)A beam of light is passed through a colloidal solution of As2S3.
(b)An electrolyte (NaCl) is added to ferric hydroxide sol.
(c)An electric current is passed through a colloidal solution.
Answer. (a)When a beam of light is passed through a colloidal solution of As2S3, the path of light becomes visible due to the scattering of light by dispersed phase particles in colloidal range. This is called Tyndal effect.
(b) The colloidal particles get precipitated i.e. ferric hydroxide is precipitated.
(c)When electric current is passed through a colloidal solution, his colloidal particles move towards oppositely charged electrodes where they lose their charge and get coagulated. This is known as electrophoresis.
Question. State what you will observe when
(i) An electrolyte is added to a colloidal solution.
(ii) An electric current is passed through a colloidal solution.
(iii) A beam of light is passed across pure water and then across a colloidal solution of sulphur when you are not in the path of light.
Answer. (i)The addition of electrolyte causes coagulation of the colloidal solution. For example, when a solution of K2SO4 is added to the ferric hydroxide (positively charged) sol, the sol gets coagulated by the negatively charged SO4–2 ions furnished by the added electrolyte.
(ii) The charged colloidal particles move towards the oppositely charged electrode on passing electric current through the colloidal solution.
(iii) The path of the beam of light is not visible through pure water but it becomes visible the colloidal solution of sulphur due to the scattering of light by colloidal particels (Tyndal effect)
Question. Explain the following terms.
(i) Peptization
(ii) Dialysis
(iii) Hardy-Schulze rule
Answer. (i) Peptization or Deflocculation is the process responsible for the formation of converting precipitate into colloid by shaking with it an electrolyte. This is particularly important in colloid chemistry or for precipitation reactions in an aqueous solution.
FIVE MARK QUESTION
Question. Show graphically how the amount of a gas absorbed on a solid in physical adsorption varies with (i) pressure and (ii) temperature?
Answer.