Students should refer to Worksheets Class 11 Chemistry The s-Block Elements Chapter 10 provided below with important questions and answers. These important questions with solutions for Chapter 10 The s-Block Elements have been prepared by expert teachers for Class 11 Chemistry based on the expected pattern of questions in the class 11 exams. We have provided Worksheets for Class 11 Chemistry for all chapters on our website. You should carefully learn all the important examinations questions provided below as they will help you to get better marks in your class tests and exams.
The s-Block Elements Worksheets Class 11 Chemistry
Question. Beryllium shows diagonal relationship with
(a) Mg
(b) Na
(c) Al
(d) B.
Answer
C
Question. Alkali metals give a _________ when dissolved in liquid ammonia
(a) Deep blue solution
(b) Colourless
(c) Red colour
(d) None of the Above
Answer
A
Question. Which elements of s- block are largely found in biological fluids?
(a) Sodium, Potassium
(b) Magnesium and Calcium
(c) Both (1) and (2) are true
(d) None of the Above
Answer
C
Question. A nitrate of an alkali metal M on heating gives O2.NO2 and M2O. The metal M will be:
(a) Na
(b) K
(c) Rb
(d) Li
Answer
D
Question. The reaction of Cl2 with X gives bleaching powder X is
(a) CaO
(b) Ca(OH)2
(c) Ca(OCl)2
(d) Ca(O3Cl)2
Answer
C
Question. Which of the following metals is not manufactured by electrolysis ?
(a) Na
(b) Mg
(c) Al
(d) Fe
Answer
D
Question. Which of the following metal carbonates decompose on heating?
(a) LiCO3 & MgCO3
(b) Na2CO3
(c) K2CO3
(d) None of the Above
Answer
A
Question. _______ does not exhibit coordination number more than four.
(a) Magnesium
(b) Beryllium
(c) Calcium
(d) None of the Above
Answer
B
Question. he wire of flash bulb is made up of:
(a) Mg
(b) Ag
(c) Cu
(d) Ba
Answer
A
Question. What are the products formed when Li2CO3 undergoes decomposition?
(a) Li2O2, CO
(b) Li2O, CO
(c) Li2O, CO2
(d) Li22, CO
Answer
C
Question. Which of the following does not illustrate the anomalous properties of Li?
(a) The m.pt. and b.pt. of Li are comparatively high.
(b) Li forms a nitride Li3N unlike group 1 metals.
(c) Li is much softer than the other group 1 metals.
(d) Li+ ion and its compounds are more heavily hydrated than those of the rest of the group.
Answer
C
Question. Magnesium forms Mg2+ and not Mg+ because
(a) magnesium(II) carbonate is insoluble in water
(b) generally higher oxidation states are preferred by metals
(c) ionic radius of Mg(II) is smaller than of Mg(I)
(d) hydration energy of divalent magnesium ion is higher.
Answer
D
Question. Lattice energies of BeF2, MgF2, CaF2 and BaF2 are –2906, – 2610, – 2459 and –2367 kJ mol–1 respectively. Hydration energies of Be2+, Mg2+, Ca2+, Ba2+ and F– are –2494, –1921, – 1577, –1305 and – 457 kJ mol–1 respectively. Which of the fluorides is soluble in water?
(a) BeF2
(b) MgF2
(c) CaF2
(d) BaF2
Answer
D
Question. The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongstfluorides of alkali metals, the lowest solubility of LiF in water is due to
(a) ionic nature of lithium fluoride
(b) high lattice enthalpy
(c) high hydration enthalpy for lithium ion
(d) low ionisation enthalpy of lithium atom.
Answer
B
Question. Strongest reducing agent in the aqueous solution is
(a) Na
(b) Rb
(c) Ca
(d) Li
Answer
D
Question. The hydroxide, which is best soluble in water, is
(a) Sr(OH)2
(b) Ba(OH)2
(c) Ca(OH)2
(d) Mg(OH)2
Answer
B
Question. Which of the following does not show diagonal relationship between beryllium and aluminium?
(a) Both BeO and Al2O3 are amphoteric in nature.
(b) Both beryllium and aluminium form polymeric covalent hydrides.
(c) Both beryllium and aluminium form nitrides with nitrogen which evolve NH3 with water.
(d) Both metal carbonates are highly stable.
Answer
D
Question. Match the column I with column II and mark the appropriate choice.

(a) (A) → (iv), (B) → (i), (C) → (iii), (D) → (ii)
(b) (A) → (i), (B) → (iii), (C) → (ii), (D) → (iv)
(c) (A) → (iii), (B) → (ii), (C) → (i), (D) → (iv)
(d) (A) → (iv), (B) → (i), (C) → (ii), (D) → (iii)
Answer
D
Question. Beryllium and aluminium exhibit many properties which are similar. But the two elements differ in
(a) maximum covalency in compounds
(b) exhibiting amphoteric nature in their oxides
(c) forming covalent halides
(d) forming polymeric hydrides.
Answer
A
Question. In the given reactions,
Be2C + H2O → BeO + X
CaC2 + H2O → Ca(OH)2 + Y
Mg2C3 + H2O → Mg(OH)2 + Z
X, Y and Z are respectively
(a) CH4, C2H2, C3H8
(b) CH4, C2H6, C3H8
(c) CH4, C2H2, C3H4
(d) C2H2, C2H6, C3H4
Answer
C
Question. If Na+ ion is larger than Mg2+ ion and S2– ion is larger than Cl– ion, which of the following will be least soluble in water?
(a) Sodium chloride
(b) Sodium sulphide
(c) Magnesium chloride
(d) Magnesium sulphide
Answer
D
Question. The reducing power of a metal depends on various factors. Suggest the factor which makes Li, the strongest reducing agent in aqueous solution.
(a) Sublimation enthalpy
(b) Ionisation enthalpy
(c) Hydration enthalpy
(d) Electron- gain enthalpy
Answer
C
Question. The activity of alkaline earth metals as reducing agents
(a) decreases from Be to Ba
(b) increases from Be to Ba
(c) increases from Be to Ca and decreases from Ca to Ba
(d) decreases from Be to Ca and increases from Ca to Ba.
Answer
B
Question. Alkali metals form hydrated compounds. The hydration enthalpies of alkali metals is in the sequence
(a) Rb+ > Li+ > Na+ > K+ > Cs+
(b) Cs+ > Rb+ > K+ > Na+ > Li+
(c) Li+ > Na+ > K+ > Rb+ > Cs+
(d) K+ > Na+ > Li+ > Rb+ > Cs+
Answer
C
Question. Which of the following is incorrect?
(a) Both BeCl2 and AlCl3 have bridged chloride structure.
(b) Both BeCl2 and AlCl3 are strong Lewis acids.
(c) Both BeCl2 and AlCl3 are covalent compounds.
(d) BeCl2 is weak Lewis acid while AlCl3 is strong Lewis acid.
Answer
D
Question. Which of the following alkali metals gives hydrated salts?
(a) Li
(b) Na
(c) K
(d) Cs
Answer
A
Question. The alkali metals have low melting points. Which of the following alkali metals is expected to melt if the room temperature rises to 30°C?
(a) Na
(b) K
(c) Rb
(d) Cs
Answer
D
Question. A metal salt solution forms a yellow precipitate with potassium chromate in acetic acid, a white precipitate with dilute sulphuric acid but does not give precipitate with sodium chloride or iodide. The white precipitate obtained when sodium carbonate is added to the metal salt solution will consist of
(a) lead carbonate
(b) basic lead carbonate
(c) barium carbonate
(d) strontium carbonate.
Answer
C
Question. The formula of nitre is
(a) KNO3
(b) NaNO2
(c) BaCl2
(d) Na2CO3
Answer
A
Question. The element A burns in nitrogen to give an ionic compound B. The compound B reacts with water to give C and D. A solution of C becomes milky on bubbling carbon dioxide. What is the nature of compound D?
(a) Acidic
(b) Basic
(c) Amphoteric
(d) Neutral
Answer
B
Case Based MCQs :
Alkali metals have the lowest ionization energy in their corresponding period in periodic table because they have large size which results in a large distance between the nucleus and the outermost electron. Ionization energy of alkali metals decreases from Li to Cs due to increase in atomic size. First ionization energy of alkali metals is very low but they have very high value of second ionization energy.
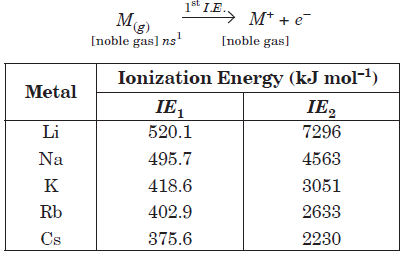
Question. Metals dissolve in liquid ammonia giving coloured solutions which are conducting in nature. The colour of the solution and reason of its conductance is
(a) yellow, NH4+
(b) blue, ammoniated metals
(c) orange, [M(NH3)x]+
(d) blue, ammoniated electron.
Answer
D
Question. Alkali metals displace hydrogen from water forming bases due to the reason that
(a) they are far above the hydrogen in electrochemical series based on oxidation potential
(b) they are far below the hydrogen in electrochemical series based on oxidation potential
(c) their ionization potential is less than that of other elements.
(d) they contain only one electron in their outermost shell.
Answer
B
Question. Alkali metals are characterised by
(a) good conductors of heat and electricity
(b) high melting points
(c) low oxidation potentials
(d) high ionisaiton potentials.
Answer
A
Alkaline earth metals are less reactive with water as compared to alkali metals. Their reactivity with water increases down the group. Be does not react with water at all, magnesium reacts only with hot water while other metals Ca, Sr and Ba react with cold water.
Order of the reactivity with water :
Ba > Sr > Ca > Mg
Be(OH)2 is amphoteric, but the hydroxides of Mg, Ca, Sr and Ba are basic. The basic strength increases from Mg to Ba.
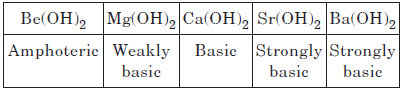
Question. What is X in the following reaction?
MgCl2 + 2H2O → X + 2HCl + H2O
(a) MgO
(b) Mg
(c) Mg(OH)2
(d) Mg(OH)Cl
Answer
A
Question. Which of the following statements is false?
(a) Strontium decomposes water readily than beryllium.
(b) Barium carbonate melts at a higher temperature than calcium carbonate.
(c) Barium hydroxide is more soluble in water than magnesium hydroxide.
(d) Beryllium hydroxide is more basic than barium hydroxide.
Answer
D
Question. Chemical compound ‘A’ is used as flocculant in water and sewage treatment. It reacts with Na2CO3 to generate caustic soda. When CO2 is bubbled through ‘A’, it turns cloudy. What is the chemical formula of ‘A’ ?
(a) CaCO3
(b) CaO
(c) Ca(OH)2
(d) Ca(HCO3)2
Answer
C
All alkali metals dissolve and form blue solution in liquid ammonia. When alkali metals are dissolved in liquid ammonia, there is a considerable expansion in total volume hence such solutions are called expanded metals. The
blue solution of an alkali metal in ammonia shows certain characteristic properties which are explained on the basis of formation of ammoniated (solvated) metal cations and ammoniated electrons in the metal ammonia solution in the following way :
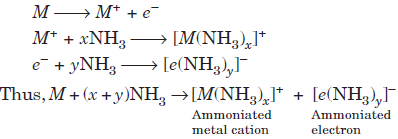
The blue solution is paramagnetic and has high electrical conductivity due to the presence of unpaired electron in the cavities in ammoniacal solution.
Question. The increasing order of the density of alkali metals is
(a) Li < K < Na < Rb < Cs
(b) Li < Na < K < Rb < Cs
(c) Cs < Rb < Na < K < Li
(d) Cs < Rb < K < Na < Li
Answer
A
Question. The reaction between sodium and water can be made less vigorous by
(a) lowering the temperature
(b) adding a little alcohol
(c) amalgamating sodium
(d) adding a little acetic acid.
Answer
C
Question. A metal M reacts with N2 to give a compound ‘A’ (M3N). ‘A’ on heating at high temperature gives back ‘M’ and ‘A’ on reacting with H2O gives a gas ‘B’. ‘B’ intensifies colour of CuSO4 solution when passes through it. M and B can be
(a) Mg and NH3
(b) Na and NH3
(c) Li and NH3
(d) Al and NH3
Answer
C
Question. Sodium dissolves in liquid NH3 to give a deep blue solution. This is due to
(a) ammoniated Na+
(b) ammoniated Na–
(c) formation of Na+/Na– pair
(d) ammoniated electrons.
Answer
D


