Worksheet on Atoms and Molecules Class 9 are one of the best study materials that students can get as it will help them to concentrate appropriately and decrease any pressure that they face before attending the exam paper.
We have provided you with Atoms and Molecules Class 9 Worksheet with answers PDF with answers for the CBSE board exam in free downloadable PDF. so students can rehearse it for their studies and get better marks in their board exam.
Students should practice questions given in Atoms And Molecules Chapter 3 Class 9 Science Worksheets. These worksheets for Class 9 Science have a good collection of important questions and answers which are expected to come in your class tests and examinations. You should learn these solved worksheet questions for Science Class 9 as it will help you to understand all topics and give you more marks.
Class 9 Science Worksheets Chapter 3 Atoms And Molecules
Please refer to below questions and answers for Atoms And Molecules Chapter 3 Class 9 Science Worksheets. Prepared by expert teachers for Standard 9 Science
Question. Write the value of charge of electron.
Answer
1.6 × 10–19 coulomb.
Question. Who stated the Law of Constant Proportion
Answer
Joseph Louis Proust stated the Law of Constant Proportion.
Question. What is meant by the term chemical formula?
Answer
The chemical formula of a compound is a symbolic representation of its composition and actual number of atoms in one molecule of a pure substance may be an atom or a compound.
Question.Name the instrument which produces image of the surface of element that shows atoms
Answer
Scanning tunnelling microscope.
Question. Write the symbols of tungsten and iron.
Answer
(i) Tungsten (W) and
(ii) Iron (Fe)
Question.Give the derivation source of symbol of sodium (Na).
Answer
The symbol of ‘Na’ for sodium is derived from its Latin name ‘Natrium’.
Question. What is the atomicity of argon?
Answer
Mono atomic.
Question. What is the latest short form of atomic mass unit?
Answer
The latest short form of atomic mass unit is u,according to IUPAC.
Question. Name any two monatomic atoms.
Answer
Sodium, Aluminium.
Question. Give difference between 2H and H2.
Answer
2H indicates 2 atoms of hydrogen and H2 indicates one molecule of hydrogen.
Question.Give two examples of triatomic molecules.
Answer
Carbon dioxide (CO2) and water (H2O).
Question. Define valency.
Answer
The combining power of an element to attain the noble gas configuration is called valency. Or, it is defined as number of electrons lost or gained by an atom to acquire noble gas configuration.
Question. Which organisation approves the names of elements all over the world?
Answer
International Union of Pure and Applied Chemistry (IUPAC).
Question. Who introduced the word ‘Mole’?
Answer
‘Wilhelm Ostwald’ introduced the word ‘Mole’.
Question. What is Avogadro Constant?
Answer
The number of particles present in one mole of any substance is fixed with a value of 6.022 × 1023.
Question. How does an atom exist?
Answer
Atom exists in the form of atom, molecule or ions.
Question. Name the element which is used as the reference for atomic mass.
Answer
Carbon.
Question. Give Latin name of Silver.
Answer
Latin name of Silver is ‘Argentum’.
Question. What is the symbol of the element of molybdenum?
Answer
‘Mo’ is the symbol of the element of molybdenum.
Question. Give an example in each of the following cases :
(i) a divalent anion
(ii) a trivalent cation
(iii) a mono-valent anion.
Answer
(i) O2– (ii) Fe3+ (iii) I–
Question. What is ion?
Answer
An ion is a charged particle. It can be positive or negative.
Question. What do you mean by symbols of elements?
Answer
Each element is represented by a letter or group of two letters to write the chemical reactions conveniently. It is called symbol.
Question. Who was the first scientist to give the concept of formation of compounds?
Answer
Antoine L. Lavoisier gave the concept of formation of compounds.
Question. Give the symbol of copper, silver, gold, oxygen, zinc.
Answer
Copper – Cu
Silver – Ag
Gold – Au
Oxygen – O
Zinc – Zn
Question. What is the difference between an atom and molecule?
Answer
An atom is the smallest particle of an element which may or may not have independent existence. For example : Na, Al, Fe, etc.
Molecule is the smallest particle of the element o compound which can exist independently. For example : O2, H2, N2, etc.
Question. Name the two laws of chemical combination.
Answer
Law of conservation of mass and law of constant proportions.
Question. Name two elements which have same atomic number.
Answer
Two elements cannot have the same atomic number.
Question. An element has 8 electrons in its valence shell. What is its general name?
Answer
Noble gas.
Question. What is meant by a chemical formula? Give examples.
Answer. A chemical formula of a compound shows its constituent elements and the number of atoms of each combining element, e.g., chemical formula of ammonia is NH3, water is H2O and carbon dioxide is CO2.
Question. Calculate the ratio of the numbers of atoms for magnesium sulphide.
Answer.
Atomic mass of Mg 3/24 = 1/8
Atomic mass of S 4/32 = 1/8
Ratio of atoms in MgS =1/8 : 1/8 or 1 : 1
Question. Why the number of atoms in one mole of hydrogen gas is double the number of atoms in one mole of helium gas? Explain.
Answer. The number of atoms in one mole of hydrogen gas is double the number of atoms in one mole of helium gas because hydrogen molecule is diatomic, i.e., a molecule of hydrogen consists of two atoms of hydrogen, whereas helium is monatomic.
Question. Give the chemical name, chemical formulae for the following : Washing soda, blue vitriol, baking soda, green vitriol, oil of vitriol, soda ash, marble chips,lime water.
Answer.

Question. Give one example each of (i) Monovalent cation, (ii) Bivalent cation, (iii) Monovalent anion, (iv) Bivalent anion.
Answer.
(i) K+ or Na+
(ii) Mg+2 or Ca+2
(iii) F– or Cl–
(iv) O2– or S2–
Question. What is Avogadro number?
Answer. The number 6.022 × 1023 is referred to as Avogadro number and is denoted by symbol NA.
Question. What are ionic compounds?
Answer. Ionic compounds are charged particles. Such compounds form by joining or losing or sharing the electron. For example : Sodium chloride is an ionic compound. Its constituent particles are positively charged sodium ion (Na+) and negatively charged chloride ion (Cl–).
Question. State two examples in each case and write their chemical formulae :
(a) Molecules having same kind of atoms only.
(b) Molecules having two different kinds of atoms.
(c) Molecules having three different kinds of atoms.
Answer.
(a) F2, Cl2, P4, S8
(b) Ammonia (NH3), Sulphur dioxide (SO2), Carbon disulphide (CS2).
(c) Calcium sulphate (CaSO4), Sodium nitrate (NaNO3).
Question. What is the difference between hydrogen chloride and nitrogen molecule formation?
Answer. Hydrogen chloride is molecular compound and formed by the union of different kinds of atoms while nitrogen is diatomic molecule and formed by union of two atoms of same kinds.
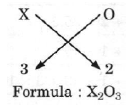
Question. If the valency of an element X is 3, then write the chemical formula of its oxide.
Answer.
Question. Write the meaning of these formulae :
(i) 2O,
(ii) O2,
(iii) O3
Answer.
(i) 2O = Two atoms of oxygen
(ii) O2 = One molecule of oxygen
(iii) O3 = One molecule of ozone
Question. Write the name of the compounds : NaBr, Al2O3, CaCO3.
Answer.
NaBr = Sodium bromide
Al2O3 = Aluminium oxide
CaCO3 = Calcium carbonate
Question. Out of these Na+, K+, Al3+ and O2–, which is isoelectronic?
Answer.
Iso-electronic means species having same number of electrons.
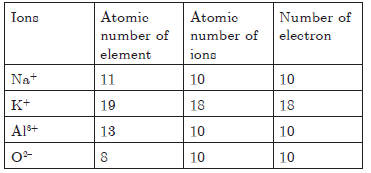
Na+, K+, Al3+ and O2– have 10 electrons each Hence, they are iso-electronic.
Question. Find the number of atoms in the 0.5 mole of C atom.
Answer.
0.5 mole of C atom :
Number of atoms in 1 mole of C atom
= 6.022 × 1023 atoms
Number of atoms in 0.5 mole of C atom
= 6.022 × 1023 × 0.5
= 3.011 × 1023 atoms
Question. Name the scientists whose experimentation established laws of chemical combination. Name the laws also.
Answer.Antoine Laurent Lavoisier and Joseph L. Proust experimented and established two laws of chemical combination.
These laws are :
(i) Law of conservation of mass,
(ii) Law of constant proportions.
Question. Write the postulate given by the Indian philosopher Maharishi Kanad.
Answer. Indian philosopher Maharishi Kanad postulated if we divide matter we will get smaller and smaller particles.He said that a time will come when we come across smallest particles beyond which further division will not be possible.
Question. Sunita calculated molecular mass of S8 molecule and reported it as 64u from the given atomic mass of 32u. But teacher considered her answer as wrong. What is the correct molecular mass of S8? Calculate the number of moles of S8 in 25.6 g of the sample.
Answer.
Molecular mass of S8 = 32 × 8 = 256u
Moles of S8 in 25.6 g sample = 25.6/25 6 = 0.1 mole
Question. Glucose has the molecular formula C6H12O6. Calculate
(a) Its molecular mass.
(b) The number of atoms in one molecule of glucose.
(c) The number of gram molecule in 18 g of glucose.
Answer.
(a) Molecular mass of C6H12O6
= (6 × 12u) + (12 × 1u) + (6 × 16u)
= 72u + 12u + 96u = 180u
(b) The number of atoms in one molecule of C6H12O6
= 6 atoms of C + 12 atoms of H
+ 6 atoms of O
= 6 + 12 + 6 = 24 atoms
(c) Number of gram molecules

18/180=0.1
Question. What is the mass of (i) 2.5 moles of CO2 and (ii) 1 mole of water?
Answer.
(i) 1 mole of CO2 = Molecular mass expressed in grams
= 1 × 44 g
2.5 moles of CO2 = 2.5 × 44 = 110 g
(ii) Mass of the substance = Moles of substance
× Molecular mass in grams
Mass of water = 1 × 18 g = 18 g
Question. Calculate the number of H2O molecules in one drop of water having a mass of 0.05 g.
Answer.
Number of moles of H2O in 0.05 g of water
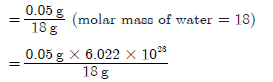
= 1.673 × 1023 molecules
Question. What is the mass percentage of different elements in calcium carbonate? (Atomic mass : Ca = 40, O = 16)
Answer.
Molecular mass of CaCO3 = At. Mass of Ca + At.
Mass of C + 3 × At. Mass of O
= 40 + 12 + 3 × 16 = 100
Mass percentage of Ca
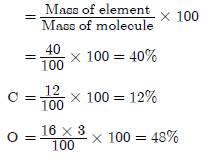
Question. What is the use of mole concept?
Answer. Applications of mole concept :
(i) We can calculate the number of basic particles from the number of moles as the number of moles of a substance is directly proportional to the number of elementary particles.
(ii) One mole of gas occupies 22.4 litres at 273K.
(iii) One mole of any gas occupies the same volume at same pressure and temperature.
(iv) One mole is equal to 6.022 × 1023 atoms. So, we can calculate the absolute masses of atoms and molecules.
Question. Define the term gram atom. How is it related to mole and Avogadro number?
Answer.
The atomic mass of an element expressed in grams is called gram atomic mass. One gram atom of any element contains 6.022 × 1023 atoms of the element. It is equal to one mole of atoms.
One gram atomic mass = 6.022 × 1023 atoms = 1 mole
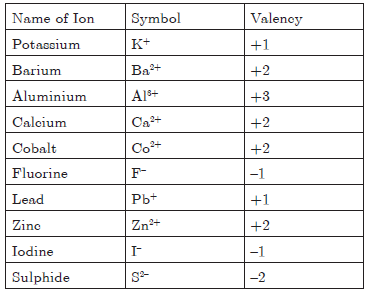
Question. Give symbol and valency of : Potassium, Barium,Aluminium, Calcium, Cobalt, Fluorine, Lead, Zinc,Iodine, Sulphide.
Answer.
Question. Ca2P2O7 is the formula of calcium pyrophosphate. Write the formula for ferric pyrophosphate.
Answer.
Valency of calcium is +2. Ca2P2O7 has two calcium atoms. So, calcium have total of +4 charges. Thus,pyrophosphate has a valency of –4. Since ferric ion has a valency of +3, the formula of ferric pyrophosphate is Fe4(P2O7)3.
Question. The mass of any single atom X is 3.05 × 10–22 g. What is its atomic weight? Name the possible element.
Answer.
1 mole = atomic mass
= 6.022 × 1023 atoms
Now, mass of one atom of X = 3.05 × 10–22 g
Mass of 6.022 × 1023 atoms of X
= 3.05 × 10–22 × 6.022 × 1023 g
= 183.7 g
This element could be tungsten.
Question. Write formula for the following :
(a) Zinc sulphate,
(b) Methane,
(c) Ammonium carbonate.
Answer.
(a) Zinc sulphate
Thus, Zn2(SO4)2 and finally = ZnSO4
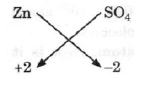
(b) Methane
Thus, finally = CH4

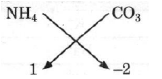
(c) Ammonium carbonate
Thus, finally = (NH4)2CO3
Question. 50 g of 10% lead nitrate is mixed with 50 g of 10% sodium chloride in a closed vessel. It was found after reaction that 6.83 g of lead chloride was precipitated.
Besides, the reaction mixture contained 90 g water and sodium nitrate. Calculate the amount of sodium nitrate formed.
Answer.
50 g of 10% lead nitrate = 5 g lead nitrate + 45 g water
50 g of 10% sodium chloride = 5 g sodium chloride + 45 g water
Total content before reaction = 5 + 5 + 90 = 100
Total content after reaction = 90 g
Amount of precipitate = 6.83 g
According to law of conservation,
Total mass of reaction mixture = 100 g
Amount of sodium nitrate = 100 – 90 – 6.83 = 3.17 g
Question. Explain the law of multiple proportions.
Answer.
According to law of multiple proportions, when two elements combine to make one or more compounds then the ratio of weights of these element remain in fixed ratio to one another. For example : Hydrogen and oxygen combine to form water (H2O) and hydrogen peroxide (H2O2) under different condition. 2 grams of hydrogen combines with 16 grams of oxygen in case of water while 2 grams of hydrogen combines with 32 grams of oxygen to form hydrogen peroxide. Now, the weights of oxygen combine with a fixed weight of hydrogen in water and hydrogen peroxide respectively are 16 and 32 which are in simple ratio of 16: 32 or 1 : 2.
Question. Explain the form of atoms in a solid.
Answer. A solid element is a cluster of atoms. The property of solid does not depend on a single atom but on cluster of atoms. For example : Diamond and graphite though both are composed of carbon atoms but due to different arrangements of carbon atoms in these.They are different in physical and chemical properties.
Worksheet on Atoms and Molecules Class 9 have been made as per the most recent CBSE schedule so there is no misstep assuming changes have been made by the CBSE board. Atoms and Molecules Class 9 Worksheet with answers have been made by educators who have great experience and know exactly what is needed.
Further, students will actually want to see a Worksheet on Atoms and Molecules class 9 PDF with short keynotes that could enhance their preparations. Atoms and Molecules Class 9 Worksheet with Answers pdf are ready with every chapter explained in a brief way from the most recent release of the books. Worksheets on Atoms and Molecules Class 9 are accessible in PDF format.