Students should refer to Worksheets Class 12 Physics Dual Nature of Radiation and Matter Chapter 11 provided below with important questions and answers. These important questions with solutions for Chapter 11 Dual Nature of Radiation and Matter have been prepared by expert teachers for Class 12 Physics based on the expected pattern of questions in the Class 12 exams. We have provided Worksheets for Class 12 Physics for all chapters on our website. You should carefully learn all the important examinations questions provided below as they will help you to get better marks in your class tests and exams.
Dual Nature of Radiation and Matter Worksheets Class 12 Physics
Question:The de Broglie wavelength of a particle of kinetic energy K is l. What will be the wavelength of the particle, if its kinetic energy is K/4 ?
(a) λ
(b) 2λ
(c)λ/2
(d)4λ
Answer:
B
Question: The threshold frequency of a certain metal is 3.3 × 1014 Hz. If light of frequency 8.2 × 1024 Hz is incident on the metal, then the cut off voltage for photoelectric emission is
(Given h = 6.63 × 10–34 J s)
(a) 2 V
(b) 4 V
(c) 6 V
(d) 8 V
Answer:
A
Question: A proton and an a-particle are accelerated through the same potential difference. The ratio of de Broglie wavelength lp to that of la is
(a) 2 : 1
(b) 4 : 1
(c) 6 : 1
(d) 8 : 1
Answer:
D
Question: A particle is dropped from a height H. The de Broglie wavelength of the particle as a function of height is proportional to
(a) H
(b) H 1/2
(c) H0
(d) H–1/2
Answer:
D
Question: Which of the following figure represents the variation of particle momentum (p) and associated de Broglie wavelength (l)?
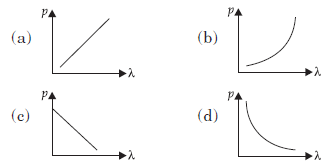
Answer:
D
Question: When the velocity of an electron increases, its de Broglie wavelength
(a) increases
(b) decreases
(c) remains same
(d) may increase or decrease
Answer:
B
Question: The wavelength of matter wave is independent of
(a) mass
(b) velocity
(c) momentum
(d) charge
Answer:
D
Question: The phenomenon of photoelectric emission was discovered in 1887 by
(a) Albert Einstein
(b) Heinrich Hertz
(c) Wilhelm Hallwachs
(d) Philipp Lenard
Answer:
B
Question: The de Broglie wavelength associated with a ball of mass 150 g travelling at 30 m s–1 is
(a) 1.47 × 10–34 m
(b) 1.47 × 10–16 m
(c) 1.47 × 10–19 m
(d) 1.47 × 10–31 m
Answer:
A
Question: If alpha particle, proton and electron move with the same momentum, then their respective de Broglie wavelengths la, lp, le are related as
(a) λa = λp =λe
(b) λa < λp < λe
(c) λa > λp > le
(d) λp > λe >λa
Answer:
A
Question: If the momentum of an electron is changed by p, then the de Broglie wavelength associated with it changes by 0.5%. The initial momentum of electron will be
(a) 200p
(b) 400p
(c) p/ 200
(d) 100p
Answer:
A
Question: Photons absorbed in matter are converted to heat. A source emitting n photons per second of frequency u is used to convert 1 kg of ice at 0°C to water at 0°C. Then, the time T taken for the conversion
(a) decreases with increasing n, with u fixed.
(b) decreases with n fixed, u increasing.
(c) remains constant with n and u changing such that nu = constant.
(d) All of these.
Answer:
D
Question: Who established that electric charge is quantised ?
(a) J.J. Thomson
(b) William Crookes
(c) R.A Millikan
(d) Wilhelm Rontgen
Answer:
C
Question: The de Broglie wavelength of an electron in a metal at 27°C is (Given me = 9.1 × 10–31 kg, kB = 1.38 × 10–23 J K–1)
(a) 6.2 × 10–9 m
(b) 6.2 × 10–10 m
(c) 6.2 × 10–8 m
(d) 6.2 × 10–7 m
Answer:
A
Question: The maximum frequency and minimum wavelength of X-rays produced by 30 kV electrons respectively is
(a) 7.24 × 1018 Hz, 0.041 nm
(b) 3.21 × 1018 Hz, 0.211 nm
(c) 5.32 × 1018 Hz, 0.001 nm
(d) 2.13 × 1018 Hz, 0.011 nm
Answer:
A
Question: A student performs an experiment on photoelectric effect using two materials A and B. A plot of stopping potential (V0)vs frequency (u) (V0) frequency is as shown in the figure. image 15
The value of h obtained from the experiment for both A and B respectively is (Given electric charge of an electron = 1.6 × 10–19 C)
(a) 3.2 × 10–34 J s, 4 × 10–34J s
(b) 6.4 × 10–344 J s, 8 × 10–34 J s
(c) 1.2 × 10–34 J s, 3.2 × 10–34 J s
(d) 4.2 × 10–34 J s, 5 × 10–34 J s
Answer:
B
Question: The minimum energy required for the electron emission from the metal surface can be supplied to the free electrons by which of the following physical processes?
(a) Thermionic emission
(b) Field emission
(c) Photoelectric emission
(d) All of these
Answer:
D
Question: Assume that a molecule is moving with the root mean square speed at temperature 300 K.
The de Broglie wavelength of nitrogen molecule is (Atomic mass of nitrogen = 14.0076 u,
h = 6.63 × 10–34 J s, kB = 1.38 × 10–23 J K–1, 1 u = 1.66 × 10–27 kg)
(a) 2.75 × 10–11 m
(b) 2.75 × 10–12 m
(c) 3.24 × 10–11 m
(d) 3.24 × 10–12 m
Answer:
A
Assertion & Reasoning Based MCQs
two statements are given-one labelled Assertion (A) and the other labelled Reason (R).
Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below.
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true but R is NOT the correct explanation of A
(c) A is true but R is false
(d) A is false and R is also false
Question: Assertion (A) : The de-Broglie wavelength of particle having kinetic energy K is λ. If its kinetic energy becomes 4 K then its new wavelength would be λ/2.
Reason (R) : The de-Broglie wavelength λ is inversely proportional to square root of the kinetic energy.
Answer
A
Question: Assertion (A) : Photoelectric effect demonstrates the wave nature of light.
Reason (R) : The number of photoelectrons is proportional to the frequency of light.
Answer
D
Question: Assertion (A) : A photon has no rest mass, yet it carries definite momentum.
Reason (R) : Momentum of photon is due to its energy and hence its equivalent mass.
Answer
A
Question: Assertion (A) : There is a physical significance of matter waves.
Reason (R) : Both interference and diffraction occurs in it.
Answer
A
Question: Assertion (A) : Photosensitivity of a metal is high if its work function is small.
Reason (R) : Work function = hu0, where u0 is the threshold frequency
Answer
B
Very Short Answer Type Questions
Question: Do all the electrons that absorb a photon come out as photoelectron?
Answer : Not all the electrons that absorb a photon come out as photoelectrons because most of electrons get scattered into the metal. Only those electrons come out as photoelectrons whose energy becomes greater than work function of metal.
Question: When a light of wavelength 400 nm falls on a metal of work function 2.5 eV, what will be the maximum magnitude of linear momentum of emitted photoelectron?
Answer:
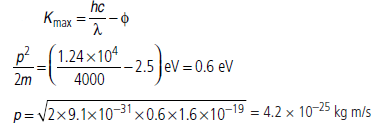
Question: If light of wavelength 412.5 nm is incident on each of the metals given in table, which one will show photoelectric emission and why? Metal Work Function (eV) 3 IMAGE
Answer : Wavelength of incident light, λ = 412.5 nm Energy of incident light, E hc /λ =1242eV nm nm/412. 5. nm= 3 eV Metals Na and K will show photoelectric emission because their work functions are less than the energy of incident light.
Question: The threshold wavelength for two photosensitive surfaces A and B areλ1 and λ2 respectively. What is the ratio of the work functions of the two surfaces?
Answer : Work function=hν=hC/λ ∴The ratio, ΦA/ΦB=hC/λAXλB/hC=λ2/λ1
Question: Write the relationship of de-Broglie wavelength l associated with a particle of mass m in terms of its kinetic energy E.
Answer : λ = h/√2mE
Short Answer Type Questions
Question: A metallic surface is irradiated with monochromatic light of variable wavelength.
Above a wavelength of 5000 Å, no photoelectrons are emitted from the surface. With an unknown wavelength, stopping potential is 3 V. Find the unknown wavelength.
Answer : According to question, λth = 5000 Å and Vs = 3V.
Using equation of photoelectric equation,
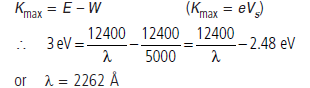
Question: (i) In the explanation of photoelectric effect, we assume one photon of frequency u collides with an electron and transfers its energy. This leads to the equation for the maximum energy
Emax of the emitted electron as
Emax = hu – φ0 where φ0 is the work function of the metal. If an electron absorbs 2 photons (each of frequency u) what will be the maximum energy for the emitted electron?
(ii) Why is this fact (two photon absorption) not taken into consideration in our discussion of thestopping potential?
Answer:
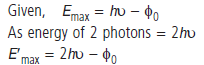
As there is one to one interaction, probability of absorbing 2 photons by same electron is very low. Thus, emission of two photon absorption is negligible.
Question: Number of photons falling in one second = P/Eλ=Pλ/ hc where P is power of light and Eλ is energy of photon.
Number of photoelectron emitted per second =Pλ/hc⋅1/106
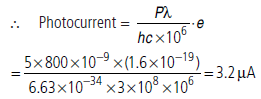
Answer : In an experiment on photoelectric effect, light of wavelength 800 nm (less than threshold wavelength) is incident on a cesium plate at the rate of 5.0 W. The potential of the collector plate is made sufficiently positive with respect to the emitter so that the current reaches its saturation value. Assuming that on the average one of every 106 photons is able to eject a photoelectron, find the photo current in the circuit.
Question: The magnetic field at a point associated with a light wave is B = 2 × 10–6 tesla sin[(3.0 × 1015 s–1)t]
sin[(6.0 × 1015 s–1)t]. If this light falls on a metal surface having a work function of 2.0 eV, what will be the maximum kinetic energy of the
photoelectrons?
Answer:

Question: A light beam of wavelength 400 nm is incident on a metal plate of work function of 2.2 eV. An electron absorbs a photon and makes some collisions before coming out of the metal.
Assuming that 10% of the instantaneous energy is lost to the metal in each collision.
(i) Find the kinetic energy of electron which makes two collisions as it comes out of the metal.
(ii) Under the same assumptions, find the minimum number of collisions the electron can suffer before it becomes unable to come out of metal.
(Use hc = 12400 eV Å)
Answer: 16 E λ = Energy of the photon = hc/= 1 .24 ×104/4000 eV = 3.1 eV
(i) K.E. of emitted e– = (3.1 × (0.9)2 – 2.2) eV = 0.31 eV
(ii) For e– not be able to come out, its energy should be less
than 2.2 eV
i.e., (3.1) × (0.9)n < 2.2, n > 4
Question: When light of wavelength l is incident on a metal surface, stopping potential is found to be x.
When light of wavelength nλ is incident on the same metal surface, stopping potential is found to be x/n +1Find the threshold wavelength ofthe metal.
Answer: Let λ0 is the threshold wavelength, the work function is
Φ=hc/λO
Now, by photoelectric equation, eVs hc/λ -hc/λ
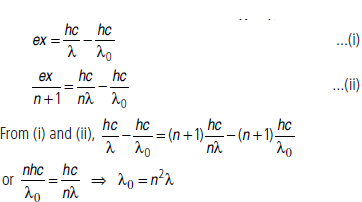
Question: Consider figure for photoemission.
metal light electron How would you reconcile with momentum-conservation? Note light (photons) have momentum in a different direction than the emitted electrons.

Answer: The momentum of incident photon is transferred to the metal during photoelectric emission at macroscopic level. At microscopic level, atoms of metal absorb photons and momentum is transferred mainly to the nuclei and the electron. Excited electrons are emitted in the process and thus momentum is conserved.
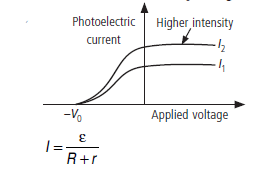
Question: (a) Define the terms, (i) threshold frequency and (ii) stopping potential in photoelectric effect.
(b) Plot a graph of photocurrent versus anode potential for a radiation of frequency u and intensities I1 and I2 (I1 < I2).
Answer: (a) (i) Threshold Frequency : The minimum frequency of incident light which is just capable of ejecting electrons from a metal is called the threshold frequency. It is denoted by u0.
(ii) Stopping Potential : The minimum retarding potential applied to anode of a photoelectric tube which is just capable of stopping photoelectric current is called the stopping potential. It is denoted by V0 (or VS).
(b)
Question: Using the graph shown in the figure for stopping potential versus the incident frequency of photons, calculate Planck’s constant.
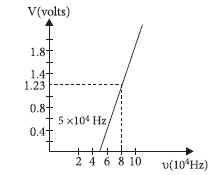
Answer: Using Einstein’s photoelectric equation,eV = hu –Φ
on differentiation we get eΔV = hΔu
or h=eΔ V /Δu =1.6 10-19X (1. 23- 0)/(8- 5)X 1014=6.56X10-34 . J s
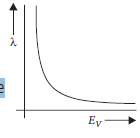
Question: X-rays fall on a photosensitive surface to cause photoelectric emission. Assuming that the work function of the surface can be neglected,find the relation between the de-Broglie wavelength (λ) of the electrons emitted and the energy (Ev) of the incident photons. Draw the nature of the graph for l as a function of Ev.
Answer: According to Einstein’s photoelectric effect
E =W +1/2 mv 2
Since work function of the surface is negligible, the above
equation becomes
E = 1/2mv 2
mv = √2mE
If λ is de-Broglie wavelength of the
emitted electrons, then
λ= h/mv= h/√2mE
Question: A beam of light consists of four wavelengths
4000 Å, 4800 Å, 6000 Å and 7000 Å, each of
intensity 1.5 × 10–3 Wm–2. The beam falls
normally on an area 10–4 m2, of a clean metallic surface of work function 1.9 eV. Assuming no loss of light energy (i.e., each capable photon emits one electron), calculate the number of photoelectrons liberated per second.
Answer:

Energy of photon (E4) is less than work function. Therefore,
light of wavelengths 4000 Å, 4800 Å,and 6000 Å can only
emit photoelectrons.
∴ Number of photoelectrons emitted per second = Number
of photons incident per second
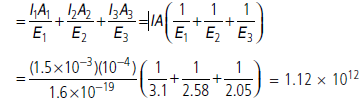
Long Answer Type Questions
Question: Consider a thin target (10–2 m square, 10–3 m thickness) of sodium, which produces a photocurrent of 100 mA when a light of intensity 100 W m–2 (l = 660 nm) falls on it.
Find the probability that a photoelectron is produced when a photon strikes a sodium atom.
[Take density of Na = 0.97 kg m–3].
Answer:

Let n be the number of photons falling per second on the
target.
Energy of each photon =hc/λ
Total energy falling per second on target
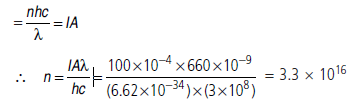
Number of electrons emitted per second by all the atoms in
the target if one electron is emitted by each atom for one
incident photon.
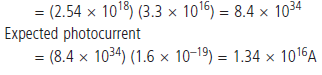
Observed photocurrent = 100 µA
Probability of photo emission by single photon incident on
a single atom
P =100µ A/1. 34 1016 A =7.5X10-21
Thus the probability of emission by single photon on a single
atom is very much less than 1, the probability of absorption
of two photons by single atoms is negligible.
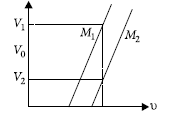
Question: Figure shows a plot of stopping potential (V0) with frequency (u) of incident radiation for two photosensitive material M1 and M2. Explain
(i) why the slope of both the lines is same?
(ii) for which material emitted electrons have greater kinetic energy for the same frequency of incident radiation?

Answer: (i) Slope of line = ΔV/ Δv[∴eΔV=hv]
Slope of line =h/e
It is a constant quantity and does not depend on nature of
metal surface.
(ii) Maximum kinetic energy of emitted photoelectron,
KE = eV0 = hu – hu0, …(i)
For a given frequency V1 > V2 (from the graph)
So from equation (i),
(KE)1 > (KE)2
Since the metal M1 has smaller threshold frequency i.e.,
smaller work function. It emits electrons having a larger
kinetic energy.
Question: A particle A with a mass mA is moving with a velocity v and hits a particle B (mass mB) at rest (one dimensional motion). Find the change in the de Broglie wavelength of the particle A.
Treat the collision as elastic.
Answer: From the law of conservation of momentum,
mAv = mAvA + mBvB
or mA(v – vA) = mBvB …(i)
(as particle B is at rest, its initial velocity is zero and vA
and vB are the velocities of particles A and B after collision)
Since the collision is elastic, kinetic energy is conserved
during collision
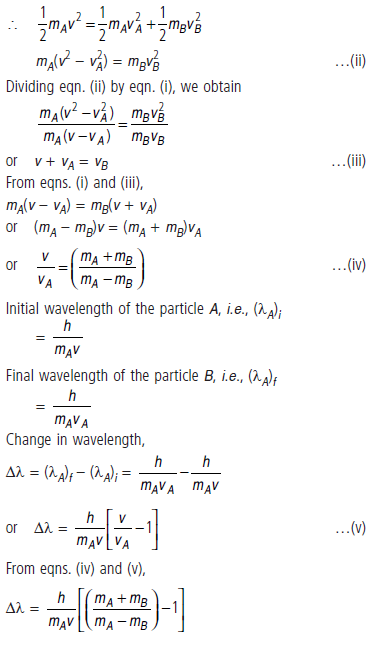
Case Based MCQs
Photoelectric Effect Photoelectric effect is the phenomenon of emission of electrons from a metal surface, when radiations of suitable frequency fall on them. The emitted electrons are called photoelectrons and the current so produced is called photoelectric current.
Question: It is observed that photoelectron emission stops at a certain time t after the light source is switched on. The stopping potential (V) can be represented as
(a) 2(KEmax/e)
(b) (KEmax/e)
(c) (KEmax/3e)
(d) (KEmax/2e)
Answer:
B
Question: A point source of light of power 3.2 × 10–3 W emits monoenergetic photons of energy 5.0 eV and work function 3.0 eV. The efficiency of photoelectron emission is 1 for every 106 incident photons. Assume that photoelectrons are instantaneously swept away after emission.
The maximum kinetic energy of photon is
(a) 4 eV
(b) 5 eV
(c) 2 eV
(d) Zero
Answer:
C
Question: With the increase of intensity of incident radiations on photoelectrons emitted by a photo tube, the number of photoelectrons emitted per unit time is
(a) increases
(b) decreases
(c) remains same
(d) none of these
Answer:
A
Question: If the frequency of incident light falling on a photosensitive metal is doubled, the kinetic energy of the emitted photoelectron is
(a) unchanged
(b) halved
(c) doubled
(d) more than twice its initial value
Answer:
D
de-Broglie Wavelength
According to de-Broglie, a moving material particle sometimes acts as a wave and sometimes as a particle or a wave associated with moving material particle which controls the particle in every respect. The wave associated with moving particle is called matter wave or de-Broglie wave where wavelength called de-Broglie wavelength, is given by λ = h/mv
Question: Which of these particles having the same kinetic energy has the largest de Broglie wavelength?
(a) Electron
(b) Alpha particle
(c) Proton
(d) Neutron
Answer:
A
Question: If a proton and an electron have the same de Broglie wavelength, then
(a) kinetic energy of electron < kinetic energy of proton
(b) kinetic energy of electron = kinetic energy of proton
(c) momentum of electron = momentum of proton
(d) momentum of electron < momentum of proton
Answer:
C
Question: Two particles A1 and A2 of masses m1, m2
(m1 > m2) have the same de Broglie wavelength.
Then
(a) their momenta are the same.
(b) their energies are the same.
(c) momentum of A1 is less than the momentum of A2.
(d) energy of A1 is more than the energy of A2.
Answer:
A
